発表論文
2022
- 三浦 裕太, 小島 憲人, 清中 茂樹, 配位ケモジェネティクスによるグルタミン酸受容体の活性制御, 日薬理誌, 157, 366-370 (2022)
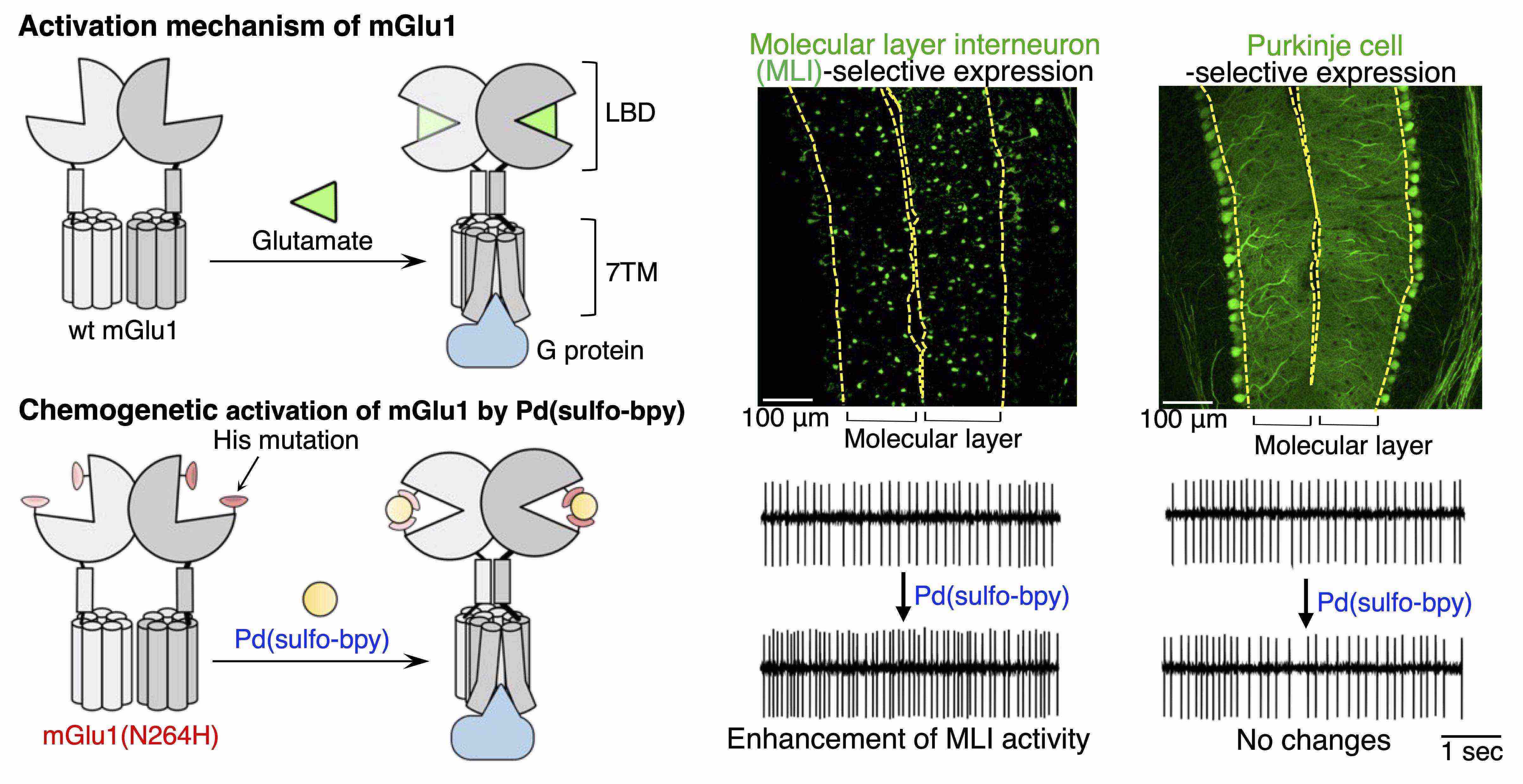
- Ojima K., Kakegawa W., Yamasaki T., Miura Y., Itoh M., Michibata Y., Kubota R., Doura T., Miura E., Nonaka H., Mizuno S., Takahashi S., Yuzaki M., Hamachi I., Kiyonaka S. Coordination chemogenetics for activation of GPCR-type glutamate receptors in brain tissue. Nat. Commun., 13, 3167 (2022). DOI: 10.1038/s41467-022-30828-0
- 杉原 佑太朗, 小島 憲人, 清中 茂樹, リガンド指向性2段階ラベル化によるAMPA型グルタミン酸受容体の精密動態解析, 日薬理誌, 157, 191-195 (2022)
- 曽我 恭平, 清中 茂樹, 迅速ケミカルラベル化法によるAMPA型グルタミン酸受容体の精密動態解析, 生化学, 94, 292-297 (2022)
- Miura Y., Senoo A., Doura T., Kiyonaka S. Chemogenetics of cell surface receptors: beyond genetic and pharmacological approaches. RSC Chem. Biol., 3, 269-287 (2022). DOI: 10.1039/d1cb00195g.
- 妹尾 暁暢, 清中 茂樹, 配位ケモジェネティクスによる代謝型グルタミン酸受容体の制御, 化学と工業, 75, 115 (2022)
- Senoo A., Yamada Y., Ojima K., Doura T., Hamachi I., Kiyonaka S. Orthogonal activation of metabotropic glutamate receptor using coordination chemogenetics. Front. Chem., 9, 825669 (2022). DOI: 10.3389/fchem.2021.825669.


2021
- 坂口 怜子, 金岡 英徳, 清中 茂樹. タンパク質温度センサーを利用した細胞内温度計測 熱測定, 48, 152-158 (2021)
- Tsai Y.H., Doura T., Kiyonaka S. Tethering-based chemogenetic approaches for the modulation of protein function in live cells. Chem. Soc. Rev., 50, 7909-7923 (2021). DOI: 10.1039/d1cs00059d.
- 三浦 裕太, 清中 茂樹, GPCRおよびGタンパク質シグナルを特異的に制御するケモジェネティクス法, 細胞, 53, 328-330 (2021).
- Ojima K., Shiraiwa K., Soga K., Doura T., Takato M., Komatsu K., Yuzaki M., Hamachi I., Kiyonaka S. Ligand-directed two-step labeling to quantify neuronal glutamate receptor trafficking. Nat. Commun., 12, 831 (2021). DOI: 10.1038/s41467-021-21082-x.
- Kojima Y., Okuzaki Y., Nishijima K., Moriwaki S., Asai S., Kaneoka H., Iijima S. Regulatory mechanism of chicken lysozyme gene expression in oviducts examined using transgenic technology. J. Biosci. Bioeng., 131, 453-459 (2021). DOI: 10.1016/j.jbiosc.2020.11.011


2020
- Hagihara Y., Okuzaki Y., Matsubayashi K., Kaneoka H., Suzuki T., Iijima S., Nishijima K. Primordial germ cell-specific expression of eGFP in transgenic chickens. Genesis., 58, e23388 (2020).
- Aoyama H., Doura T. Selective acetylcholinesterase inhibitors derived from muscle relaxant dantrolene. Bioorg. Med. Chem. Lett., 30, 126888 (2020). DOI: 10.1016/j.bmcl.2019.126888.
2019
- Tateyama H., Murase Y., Higuchi H., Inasaka Y., Kaneoka H., Iijima S., Nishijima K. Siglec-F is induced by granulocyte–macrophage colony-stimulating factor and enhances interleukin-4-induced expression of arginase-1 in mouse macrophages. Immunology, 158, 340-352 (2019). DOI: 10.1111/imm.13121
- Sakamoto S., Yamaura K., Numata T., Harada F., Amaike K., Inoue R., Kiyonaka S., Hamachi I. Construction of a Fluorescent Screening System of Allosteric Modulators for the GABAA Receptor Using a Turn-On Probe. ACS Cent. Sci., 5, 1541-1553 (2019). DOI: 10.1021/acscentsci.9b00539.
- Okuzaki Y., Kaneoka H., Suzuki T., Hagihara Y., Nakayama Y., Murakami S., Murase Y., Kuroiwa A., Iijima S., Nishijima K. PRDM14 and BLIMP1 control the development of chicken primordial germ cells. Dev. Biol., 455, 32-41 (2019). DOI: 10.1016/j.ydbio.2019.06.018
- Doura T., Nishio T., Tamanoi F., Nakamura M. Relationship between the glutathione-responsive degradability of thiol-organosilica nanoparticles and the chemical structures. J. Mater. Res., 34, 1266-1278 (2019). DOI:org/10.1557/jmr.2018.501
- Kubota R., Kiyonaka S., Hamachi I. On-cell coordination chemistry: Chemogenetic activation of membrane-bound glutamate receptors in living cells. Methods Enzymol., 622, 411-430 (2019). DOI: 10.1016/bs.mie.2019.02.033.
- Sakamoto S, Kiyonaka S, Hamachi I. Construction of ligand assay systems by protein-based semisynthetic biosensors. Curr. Opin. Chem. Biol., 50, 10-18 (2019). DOI: 10.1016/j.cbpa.2019.02.011.
- Mekaru H., Yoshigoe A., Nakamura M., Doura T., Tamanoi F. Biodegradability of disulfide-organosilica nanoparticles evaluated by soft X-ray photoelectron spectroscopy: cancer therapy implications. ACS Appl. Nano Mater. 2, 479-488 (2019). DOI: 10.1021/acsanm.8b02023