
原著論文 : Original Papers
-
111.
Deoxygenative [3 + 2] Annulation of α,β-Unsaturated Carbonyl Compounds and Electron-Rich Olefins via Photocatalytic Umpolung of Triarylphosphine
Taiga Ando, Daisuke Yokogawa, Kohsuke Ohmatsu, Takashi Ooi
J. Am. Chem. Soc. 2025, xx, xxx–xxx.

-
110.
Acceptorless Dehydrogenative Aminoalkylation of Allylic C(sp3)–H Bonds via Reversible Hydrogen Atom Transfer
Kodai Minami, Hiroki Fujita, Kohsuke Ohmatsu, Takashi Ooi
Org. Lett. 2025, 27, 6919-6923.

-
109.
Regiodivergent Photocatalytic Annulation for the Synthesis of gem-Difluorinated Cyclic Hydrocarbons
Tobias E. Schirmer, Julian C. G. Kürschner, Yuki Uchida, Yuya Taura, Pablo Gabriel, Line Næsborg, Daisuke Yokogawa, Yoshitaka Aramaki, Takashi Ooi
Angew. Chem. Int. Ed. 2025, e202502450

-
108.
Epimerization mechanism of P-spiro chiral tetraaminophosphoniums: effect of nitrogen substituents and counter ions
Yaoki Kansaku, Yoshitaka Aramaki, Daisuke Uraguchi, Takashi Ooi
Chem. Lett. 2025, 54, upaf085

-
107.
Zwitterionic Acridinium Amidate for Photocatalytic Acceptorless Dehydrogenation
Soichiro Mori, Lukas-Maximilian Entgelmeier, Yaoki Kansaku, Duc An Truong, Olga García Mancheño, Kohsuke Ohmatsu, Takashi Ooi
Synlett 2025, 36, 1739-1742.

-
106.
If the Crown Fits: Sterically Demanding N-Heterocyclic Carbene Promotes the Formation of Au8Pt Nanocluster
Joseph F. DeJesus, Samuel I. Jacob, Quan Manh Phung, Koichi Mimura, Yoshitaka Aramaki, Takashi Ooi, Masakazu Nambo, Cathleen M. Crudden
J. Am. Chem. Soc. 2024, 146, 23806-23813.

-
105.
p-Diarylboryl Halothiophenols as Multifunctional Catalysts via Photoactive Intramolecular Frustrated Lewis Pairs
Takeru Kikura, Yuya Taura, Yoshitaka Aramaki, and Takashi Ooi
J. Am. Chem. Soc. 2024, 146, 20425-20431.

-
104.
Catalytic 1,1-Cyanoalkylation of Electron-Deficient Olefins
Kohsuke Ohmatsu, Duc An Truong, Shohei Morita, Keiji Maruoka, and Takashi Ooi
Org. Lett. 2024, 26, 4055-4058.

-
103.
Zwitterionic Acridinium Amidate: A Nitrogen-Centered Radical Catalyst for Photoinduced Direct Hydrogen Atom Transfer
Lukas Entgelmeier, Soichiro Mori, Shion Sendo, Rie Yamaguchi, Ryuhei Suzuki, Takeshi Yanai, Olga García Mancheño, Kohsuke Ohmatsu, Takashi Ooi
RhemRxiv. 2023
DOI: 10.26434/chemrxive-2023-pj884
Angew. Chem. Int. Ed. 2024, e202404890

-
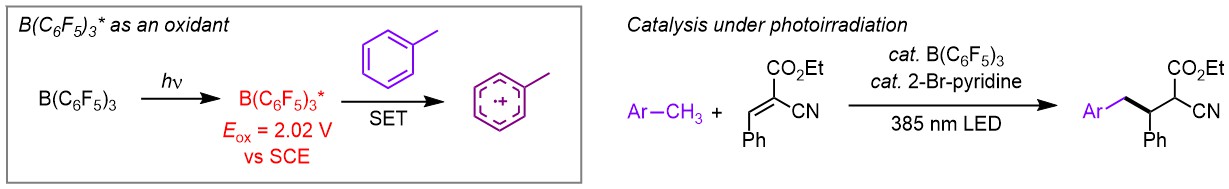
102.
Excited-state tris(pentafluorophenyl)borane as a strong single-electron oxidant: Photophysical properties and catalysis
Yoshitaka Aramaki, Yuki Uchida, Ryo Ishikawa, and Takashi Ooi
J. Photochem. Photobio. 2023, 18, 100206.
-
101.
Catalytic Formal Carbyne Transformation of Phosphorus Ylides
Ryuhei Suzuki, Taiga Ando, Fritz Deufel, Kohsuke Ohmatsu, and Takashi Ooi
ChemRxiv 2023
DOI: 10.26434/chemrxiv-2023-mx0vg
Photocatalytic carbyne reactivity of phosphorus ylides for three-component formal cycloaddition reactions
Nat. Synth. 2024, 3,1385-1391.
DOI: 10.1038/s44160-024-00612-7

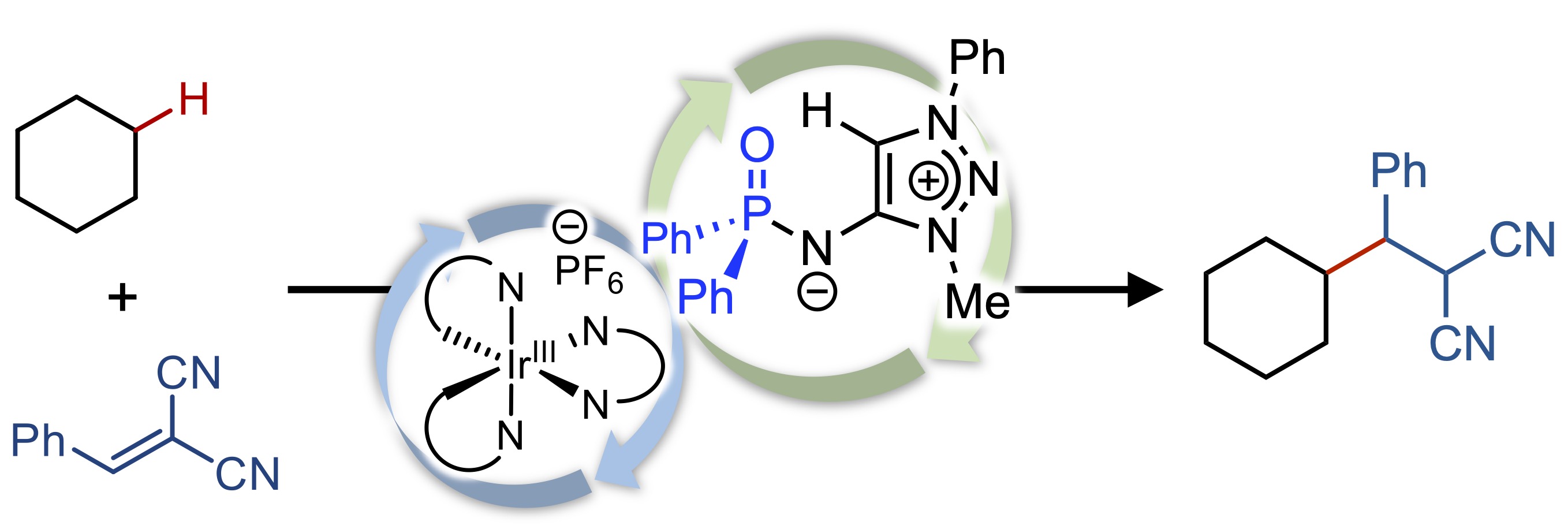
100.
Zwitterionic Diphenylphosphinyl Amidate as a Powerful Photoinduced Hydrogen-Atom-Transfer Catalyst for C-H Alkylation of Simple Alkanes
Kohsuke Ohmatsu, Ryuhei Suzuki, Hiroki Fujira, and Takashi Ooi
J. Org. Chem. 2023, 88, 6553-6556.
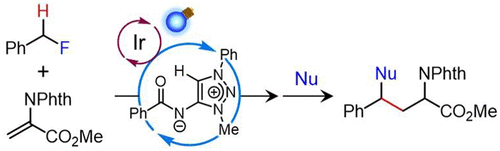
99.
Hydrogen-Atom Transfer Catalysis for C-H Alkylation of Benzylic Fluorides
Kohsuke Ohmatsu, Hiroki Fujita, Ryuhei Suzuki, and Takashi Ooi
Org. Lett. 2022, 24, 3134-3137.
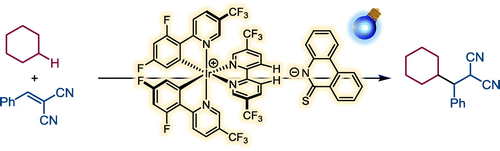
98.
Thioamidate Ion as Effective Cocatalyst for Photoinduced C-H Alkylation via Multisite Proton-coupled Electron Transfer
Kohsuke Ohmatsu, Haruka Fujimori, Kodai Minami, Kosuke Nomura, Mari Kiyokawa, and Takashi Ooi
Chem. Lett. 2022, 51, 445-447.
97.
In Silico Analysis and Synthesis of Nafamostat Derivatives and Evaluation of Their Anti-SARS-CoV-2 Activity
Kazuhiro J. Fujimoto, Daniel C. F. Hobbs, Miki Umeda, Akihiro Nagata, Rie Yamaguchi, Yoshitaka Sato, Ayato Sato, Kohsuke Ohmatsu, Takashi Ooi, Takeshi Yanai, Hiroshi Kimura, Takayuki Murata
Viruses 2022, 14, 389.

96.
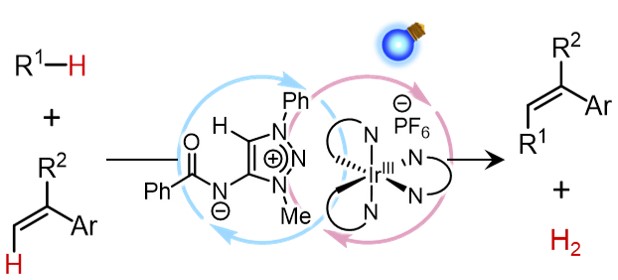
Hydrogen-Atom-Transfer-Mediated Acceptorless Dehydrogenative Cross-Coupling Enabled by Multiple Catalytic Functions of Zwitterionic Triazolium Amidate
Kodai Minami, Kohsuke Ohmatsu, and Takashi Ooi
ACS Catal. 2022, 12, 1971
95.
Catalytic Asymmetric Strecker Reaction of Ketoimines with Potassium Cyanide
Kohsuke Ohmatsu, Yusuke Morita, Mari Kiyokawa, Kimihiro Hoshino, and Takashi Ooi
Asian J. Org. Chem. 2021, 10, 3237-3240
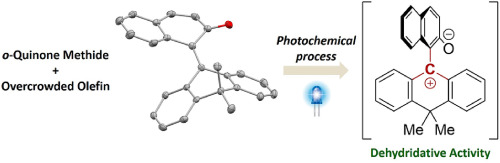
94.
o-Quinone methide with overcrowded olefinic core as a catalytically-active surrogate of triarylmethylium salt for dehydridative oxidationof benzylic alcohols under aerobic photoirradiation conditions
Kohsuke Kato, Daisuke Uraguchi, and Takashi Ooi
Tetrahedron 2021, 100, 132459
93.
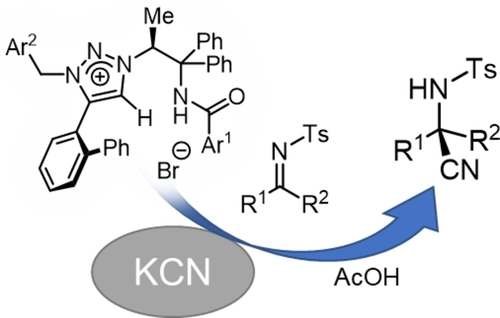
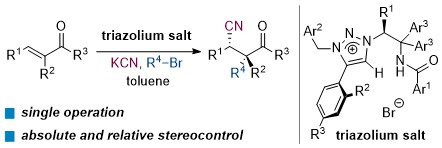
Catalytic Asymmetric Cyanoalkylation of Electron-Deficient Olefins with Potassium Cyanide and Alkyl Halides
Kohsuke Ohmatsu, Yusuke Morita, Mari Kiyokawa, and Takashi Ooi
J. Am. Chem. Soc. 2021, 143, 11218-11224
92.
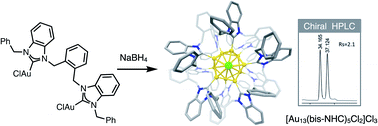
Synthesis and enantioseparation of chiral Au13 nanoclusters protected by bis-N-heterocyclic carbene ligands
Hong Yi, Kimberly M. Osten, Tetyana I. Levchenko, Alex J. Veinot, Yoshitaka Aramaki, Takashi Ooi, Masakazu Nambo, and Cathleen M. Crudden
Chem. Sci. 2021, 12, 10436-10440
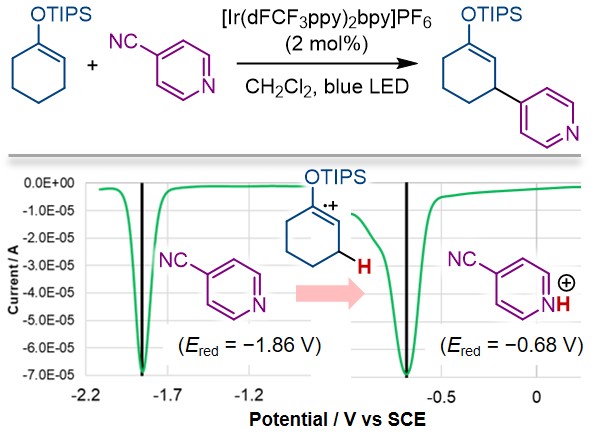
91.
Exploiting Transient Radical Cations as Brønsted Acids for Allylic C-H Heteroarylation of Enol Silyl Ethers
Tsubasa Nakashima, Haruka Fujimori, Kohsuke Ohmatsu, and Takashi Ooi
Chem. Eur. J. 2021, 27, 9253-9256
90.
Catalytic Asymmetric Synthesis of 5-Membered Alicyclic α-Quaternary β-Amino Acids via [3+2]-Photocycloaddition of α-Substituted Acrylates
Yuto Kimura, Daisuke Uraguchi and Takashi Ooi
Org. Biomol. Chem. 2021, 19, 1744-1747
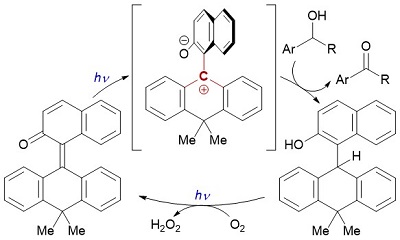
89.
o-Quinone Methide with Overcrowded Olefin Component as a Dehydridation Catalyst under Aerobic Photoirradiation Conditions
Daisuke Uraguchi, Kohsuke Kato, and Takashi Ooi
Chem. Sci. 2021, 12, 2778-2783
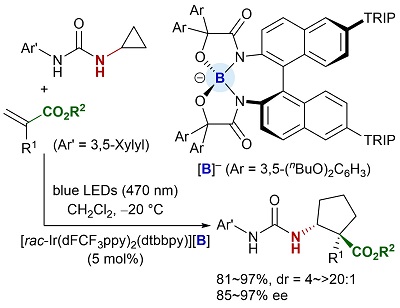
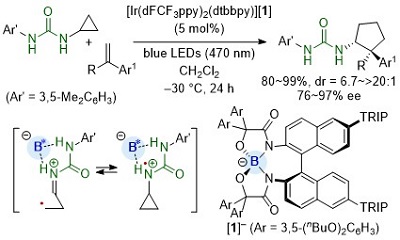
88.
Urea as a Redox-Active Directing Group under Asymmetric Photocatalysis of Iridium-Chiral Borate Ion Pairs
Daisuke Uraguchi, Yuto Kimura, Fumito Ueoka, and Takashi Ooi
J. Am. Chem. Soc. 2020, 142, 19462-19467
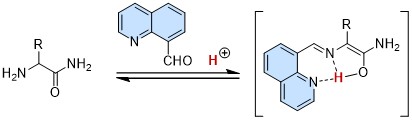
87.
Hybrid Catalysis of 8-Quinolinecarboxaldehyde and Br?nsted Acid for Efficient Racemization of α-Amino Amides and Its Application in Chemoenzymatic Dynamic Kinetic Resolution
Kohsuke Ohmatsu, Mari Kiyokawa, Yuto Shirai, Yuya Nagato, and Takashi Ooi
HETEROCYCLES 2021, 103, 218-224
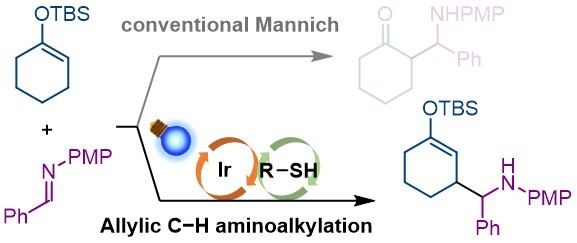
86.
Mannich-type allylic C-H functionalization of enol silyl ethers under photoredox-thiol hybrid catalysis
Tsubasa Nakashima, Kohsuke Ohmatsu, Takashi Ooi
Org. Biomol. Chem. 2021, 19, 141-145
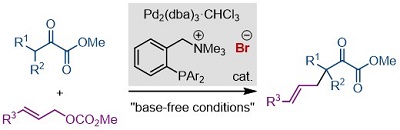
85.
Ion-Paired Ligands for Palladium-Catalyzed Allylic Alkylation under Base-Free Conditions
Kohsuke Ohmatsu, Naho Matsuyama, Yuya Nagato, and Takashi Ooi
Chem. Lett. 2020, 49, 1114-1116
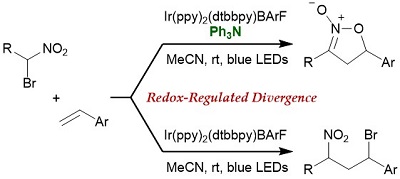
84.
Redox-Regulated Divergence in Photocatalytic Addition of α-Nitro Alkyl Radicals to Styrenes
Yuto Tsuchiya, Ryota Onai, Daisuke Uraguchi and Takashi Ooi
Chem. Commun. 2020, 56, 11014-11017
83.
A Structurally Robust Chiral Borate Ion: Molecular Design, Synthesis, and Asymmetric Catalysis
Daisuke Uraguchi, Fumito Ueoka, Naoya Tanaka, Tomohito Kizu, Wakana Takahashi, Takashi Ooi
Angew. Chem. Int. Ed. 2020, 59, 11456-11461
82.
Exploiting Single-Electron Transfer in Lewis Pairs for Catalytic Bond-Forming Reactions
Yoshitaka Aramaki, Naoki Imaizumi, Mao Hotta, Jun Kumagai, and Takashi Ooi
Chem. Sci. 2020, 11, 4305-4311
81.
Non‐Enzymatic Hybrid Catalysis for Stereoconversion of l-Amino Acid Derivatives to d-Isomers
Yuya Nagato, Mari Kiyokawa, Yusuke Ueki, Jun Kikuchi, Kohsuke Ohmatsu, Masahiro Terada, Takashi Ooi
Asian J. Org. Chem. 2020, 9, 561-565
80.
Unveiling Latent Photoreactivity of Imines
Daisuke Uraguchi, Yuto Tsuchiya, Tsuyoshi Ohtani, Takafumi Enomoto, Shigeyuki Masaoka, Daisuke Yokogawa, and Takashi Ooi
Angew. Chem. Int. Ed. 2020, 59, 3665-3670
79.
Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation
Kohsuke Ohmatsu, Ryuhei Suzuki, Yukino Furukawa, Makoto Sato, and Takashi Ooi
ACS Catal. 2020, 10, 2627-2632
78.
Inserting Nitrogen: An Effective Concept To Create Nonplanar and Stimuli-Responsive Perylene Bisimide Analogues
Sakiho Hayakawa, Ayumi Kawasaki, Yongseok Hong, Daisuke Uraguchi, Takashi Ooi, Dongho Kim, Tomoyuki Akutagawa, Norihito Fukui, and Hiroshi Shinokubo
J. Am. Chem. Soc. 2019, 141, 19807-19816
77.
Direct allylic C-H alkylation of enol silyl ethers enabled by photoredox-Brønsted base hybrid catalysis
Kohsuke Ohmatsu, Tsubasa Nakashima, Makoto Sato, and Takashi Ooi
Nat. Commun. 2019, 10, 2706
76.
Formal Hydroformylation of α,β-Unsaturated Carboxylic Acids under Photoexcited Ketone Catalysis
Kailong Zhu, Tsuyoshi Ohtani, Chandra Bhushan Tripathi, Daisuke Uraguchi, and Takashi Ooi
Chem. Lett. 2019, 48, 715-717
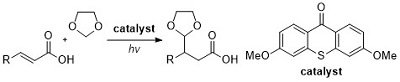
75.
Photocatalytic Borylcyclopropanation of α-Boryl Styrenes
Tsuyoshi Ohtani, Yuto Tsuchiya, Daisuke Uraguchi and Takashi Ooi
Org. Chem. Front. 2019, 6, 1734-1737
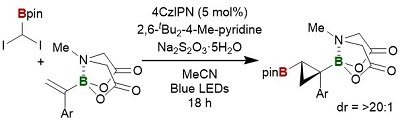
74.
A femto-molar range suicide germination stimulant for the parasitic plant Striga hermonthica
Daisuke Uraguchi, Keiko Kuwata, Yuh Hijikata, Rie Yamaguchi, Hanae Imaizumi, Sathiyanarayanan AM, Christin Rakers, Narumi Mori, Kohki Akiyama, Stephan Irle, Peter McCourt, Toshinori Kinoshita, Takashi Ooi, Yuichiro Tsuchiya
Science 2018, 362, 1301-1305
読売新聞, 朝日新聞, 中日新聞, 産経新聞, 時事通信(2018年12月14日), 毎日新聞(16日), 日経産業新聞(18日), 科学新聞(21日),現代化学2019年4月号(インタビュー) 掲載
Highlighted in Synform 2019, A74-76. http://dx.doi.org/10.1055/s-0037-1612166

73.
Protonated Bis-1,2,3-triazole as an Anion-Binding Chiral Br&onsted Acid for Catalytic Asymmetric Friedel-Crafts Reaction of Indoles with Imines
Yukino Furukawa, Ryuhei Suzuki, Tsubasa Nakashima, Rafael Gramage-Doria, Kohsuke Ohmatsu, Takashi Ooi
Bull. Chem. Soc. J. 2018, 91, 1252-1257
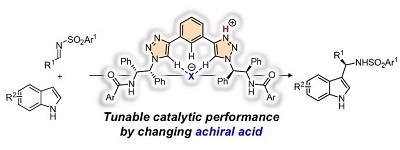
72.
Catalyst-Directed Guidance of Sulfur-Substituted Enediolates to Stereoselective Carbon-Carbon Bond Formation with Aldehydes
Daisuke Uraguchi, Kohei Yamada, Makoto Sato, and Takashi Ooi
J. Am. Chem. Soc. 2018, 140, 5110-5117
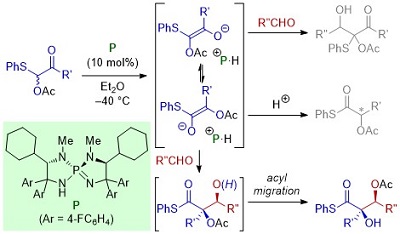
71.
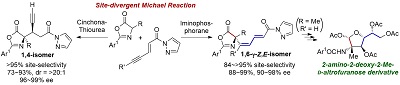
Catalyst-Enabled Site-divergent Stereoselective Michael Reactions: Overriding Intrinsic Reactivity of Enynyl Carbonyl Acceptors
Daisuke Uraguchi, Ryo Shibazaki, Naoya Tanaka, Kohei Yamada, Ken Yoshioka, and Takashi Ooi
Angew. Chem. Int. Ed. 2018, 57, 4732-4736
Selected as a "VIP"
70.
Allenedicarboxylate as a Stereochemically Labile Electrophile for Chiral Organic Base-Catalyzed Stereoselective Michael Addition
Daisuke Uraguchi, Yasutaka Kawai, Hitoshi Sasaki, Kohei Yamada, and Takashi Ooi
Chem. Lett. 2018, 47, 594-597
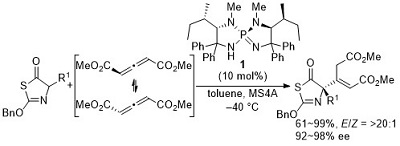
69.
Molecular Design, Synthesis, and Asymmetric Catalysis of a Hexacoordinated Chiral Phosphate Ion
Daisuke Uraguchi, Hitoshi Sasaki, Yuto Kimura, Takaki Ito, and Takashi Ooi
J. Am. Chem. Soc. 2018, 140, 2765-2768
68.
Diastereo- and enantioselective phase-transfer alkylation of 3-substituted oxindoles with racemic secondary alkyl halides
Kohsuke Ohmatsu, Yukino Furukawa, Mari Kiyokawa and Takashi Ooi
Chem. Commun. 2017, 53, 13113-13116
67.
Photoredox Ketone Catalysis for Direct C-H Imidation and Acyloxylation of Arenes
Chandra Bhushan Tripathi, Tsuyoshi Ohtani, Michael T. Corbett and Takashi Ooi
Chem. Sci. 2017, 8, 5622-5627
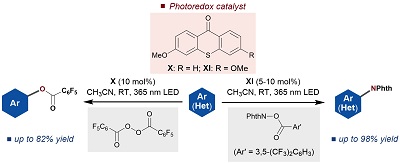
66.
Determination of the absolute configuration of compounds bearing chiral quaternary carbon centers using the crystalline sponge method
Shiho Sairenji, Takashi Kikuchi, Mohamed Ahmed Abozeid, Shinobu Takizawa, Hiroaki Sasai, Yuichiro Ando, Kohsuke Ohmatsu, Takashi Ooi and Makoto Fujita
Chem. Sci. 2017, 8, 5132-5136
65.
N-Sulfonyl α-Imino Ester-Derived Chiral Oxaziridines: Catalytic Asymmetric Synthesis and Application as a Modular Chiral Organic Oxidant
Naoya Tanaka, Ryosuke Tsutsumi, Daisuke Uraguchi,Takashi Ooi
Chem. Commun. 2017, 53, 6999-7002
64.
Unique Site-selectivity Control in Asymmetric Michael Addition of Azlactone to Alkenyl Dienyl Ketones Enabled by P-Spiro Chiral Iminophosphorane Catalysis
Ken Yoshioka, Kohei Yamada, Daisuke Uraguchi,Takashi Ooi
Chem. Commun. 2017, 53, 5495-5498
Themed Issue “Site-selective molecular transformations”
63.
Acridinium Betaine as a Single-Electron-Transfer Catalyst: Design and Application to Dimerization of Oxindoles
Daisuke Uraguchi, Masahiro Torii, Takashi Ooi
ACS Catal. 2017, 7, 2765-2769
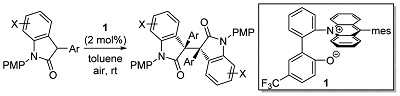
62.
Complete diastereodivergence in asymmetric 1,6-addition reactions enabled by minimal modification of a chiral catalyst
Daisuke Uraguchi, Ken Yoshioka, Takashi Ooi
Nature Communications 2017, 8, 14793
中日新聞(2017年4月4日)掲載
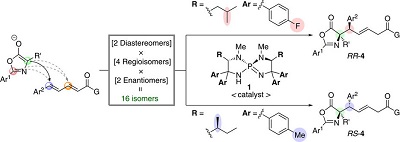
61.
[5.5]-P-Spirocyclic Chiral Triaminoiminophosphorane-Catalyzed Asymmetric Hydrophosphonylation of Aldehydes and Ynones
Daisuke Uraguchi, Takaki Ito, Yuto Kimura, Yumiko Nobori, Makoto Sato, Takashi Ooi
Bull. Chem. Soc. Jpn. 2017, 90, 546-555
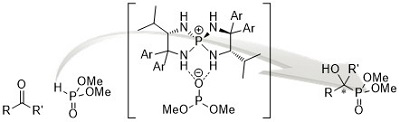
60.
In Situ Electrophilic Activation of Hydrogen Peroxide for Catalytic Asymmetric α-Hydroxylation of 3-Substituted Oxindoles
Kohsuke Ohmatsu, Yuichiro Ando, Takashi Ooi
Synlett 2017, 28, 1291-1294
SYNLETT Cluster: Asymmetric Brønsted Base Catalysis
Selected as a " News article"
Selected as a " Front Cover"
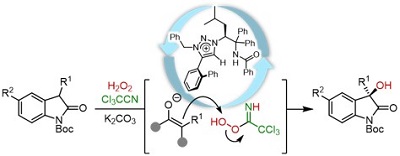
59.
Stereoselective Aza-Henry Reaction of 3-Nitro-Dihydro-2(1H)-Quinolones with N-BOC-Aldimines under the Catalysis of Chiral Ammonium Betaines
Daisuke Uraguchi, Masahiro Torii, Kohsuke Kato, Takashi Ooi
Heterocycles 2017, 94, 441-447
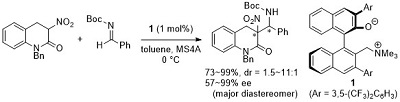
58.
A Modular Strategy for the Direct Catalytic Asymmetric α-Amination of Carbonyl Compounds
Kohsuke Ohmatsu, Yuichiro Ando, Tsubasa Nakashima, Takashi Ooi
Chem 2016, 1, 802-810
DOI: 10.1016/j.chempr.2016.10.012
Highlighted in Chem-Station (化学者のつぶやき)
中日新聞・日経産業新聞・日刊工業新聞 (2016年11月11日), 科学新聞(2016年11月25日)掲載
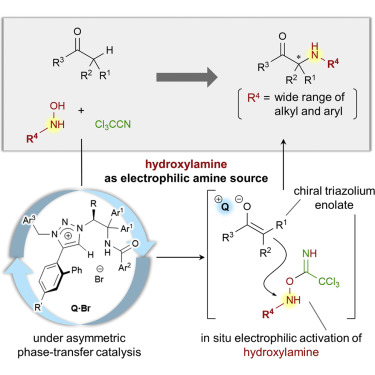
57.
Origin of High Regio-, Diastereo-, and Enantioselectivities in 1,6-Addition of Azlactones to Dienyl N-Acylpyrroles: A Computational Study
Yamanaka, Masahiro; Sakata, Ken; Yoshioka, Ken; Uraguchi, Daisuke; Ooi, Takashi
J. Org. Chem. 2017, 82, 541-548
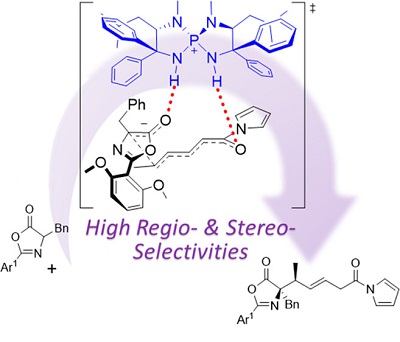
56.
Chiral Ammonium Betaine-Catalyzed Asymmetric Mannich-Type Reaction of Oxindoles
Masahiro Torii, Kohsuke Kato, Daisuke Uraguchi, and Takashi Ooi
Beilstein J. Org. Chem. 2016, 12, 2099-2103
Thematic Series “Strategies in Asymmetric Catalysis”
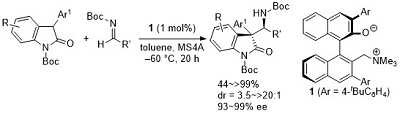
55.
Independence from the Sequence of Single-Electron Transfer of Photoredox Process in Redox-Neutral Asymmetric Bond-Forming Reaction
Tomohito Kizu, Daisuke Uraguchi, and Takashi Ooi
J. Org. Chem. 2016, 81, 6953-6958
Special Issue on Photocatalysis
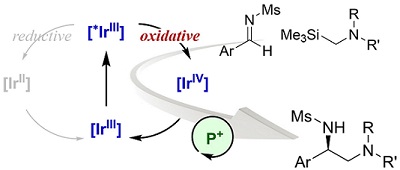
54.
Multiple Absolute Stereocontrol in Pd-Catalyzed [3+2] Cycloaddition of Oxazolidinones and Trisubstituted Alkenes Using Chiral Ammonium-Phosphine Hybrid Ligands
Naomichi Imagawa, Yuya Nagato, Kohsuke Ohmatsu, and Takashi Ooi
Bull. Chem. Soc. Jpn. 2016, 89, 649-656
Selected as a "selected as BCSJ Award Article and Cover Picture"
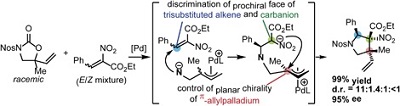
53.
Anion-Stoichiometry-Dependent Selectivity Enhancement in Ion-Paired Chiral Ligand-Palladium Complex Catalyzed Enantioselective Allylic Alkylation
Kohsuke Ohmatsu, Yoshiyuki Hara, Yuya Kusano, Takashi Ooi
Synlett 2016, 27, 1047-1050
SYNLETT Cluster: Non-Covalent Interactions in Asymmetric Catalysis
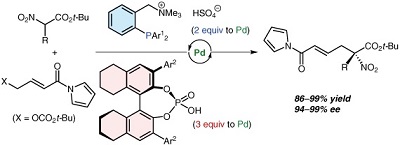
52.
Palladium-Catalyzed Branch-Selective Decarboxylative Allylation Using Ion-Paired Ligands
Yoshiyuki Hara, Yuya Kusano, Kohsuke Ohmatsu, and Takashi Ooi
Chem. Lett. 2016, 45, 552-554
Selected as a "Editor's Choice"
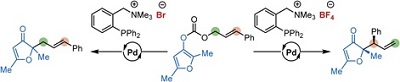
51.
Synergistic Catalysis of Ionic Brønsted Acid and Photosensitizer for a Redox Neutral Asymmetric α-Coupling of N-Arylaminomethanes with Aldimines
Daisuke Uraguchi, Natsuko Kinoshita, Tomohito Kizu, and Takashi Ooi
J. Am. Chem. Soc. 2015, 137, 13768-13771
50.
Enantioselective Reductive Multicomponent Coupling Reactions between Isatins and Aldehydes
Matthew A. Horwitz, Naoya Tanaka, Takuya Yokosaka, Daisuke Uraguchi, Jeffrey S. Johnson, and Takashi Ooi
Chem. Sci. 2015, 6, 6086-6090
49.
Catalytic Asymmetric Cyanation of Alkylideneindolenines Generated from Sulfonylalkylindoles
Kohsuke Ohmatsu, Yukino Furukawa, Daigo Nakaguro, and Takashi Ooi
Chem. Lett. 2015, 44, 1350-1352
48.
Highly E- and Enantioselective Michael Addition to Electron-deficient Internal Alkynes under the Catalysis of Chiral Iminophosphorane
Daisuke Uraguchi, Kohei Yamada, Takashi Ooi
Angew. Chem. Int. Ed. 2015, 54, 9954-9957
47.
Vinylogy in Nitronates: Utilization of α-Aryl Conjugated Nitroolefins as a Nucleophile for Highly Stereoselective Aza-Henry Reaction
Keigo Oyaizu, Daisuke Uraguchi, Takashi Ooi
Chem. Commun. 2015, 51, 4437-4439
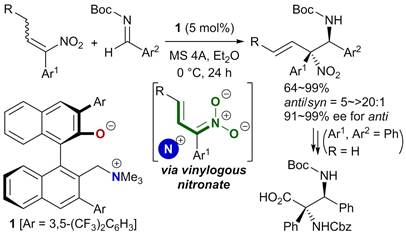
46.
Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition of 5-Vinyloxazolidinones with Imines Using Chiral Ammonium-Phosphine Hybrid Ligand
Kohsuke Ohmatsu, Shinya Kawai, Naomichi Imagawa, Takashi Ooi
ACS Catal. 2014, 4, 4304-4306
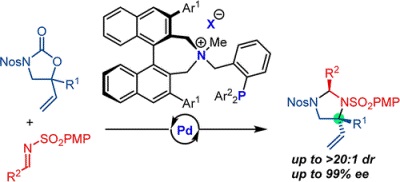
45.
Chiral Ammonium Betaine-Catalyzed Highly Stereoselective Aza-Henry Reaction of α-Aryl Nitromethanes with Aromatic N-Boc Imines
Daisuke Uraguchi, Keigo Oyaizu, Haruhiro Noguchi, Takashi Ooi
Chem. Asian J. 2014, 10, 334-337
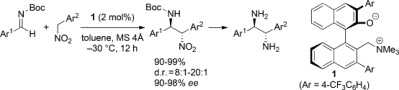
44.
Enantioselective Protonation of α-Hetero Carboxylic Acid-Derived Ketene Disilyl Acetals under Chiral Ionic Brønsted Acid Catalysis
Daisuke Uraguchi, Tomohito Kizu, Yuki Ohira, Takashi Ooi
Chem. Commun. 2014, 50,13489-13491
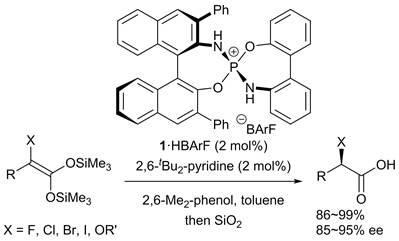
43.
In-Situ Generation of Ion-Paired Chiral Ligands: Rapid Identification of Optimal Ligand for Palladium-Catalyzed Asymmetric Allylation
Kohsuke Ohmatsu, Yoshiyuki Hara, Takashi Ooi
Chem. Sci. 2014, 5, 3645-3650
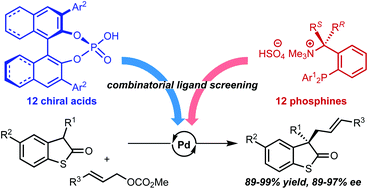
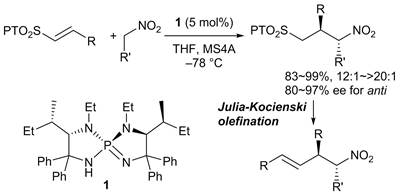
42.
Enantioselective formal α-allylation of nitroalkanes through a chiral iminophosphorane-catalyzed Michael reaction-Julia-Kocienski olefination sequence
Daisuke Uraguchi, Shinji Nakamura, Hitoshi Sasaki, Yuki Konakade, Takashi Ooi
Chem. Commun. 2014, 50, 3491-3493
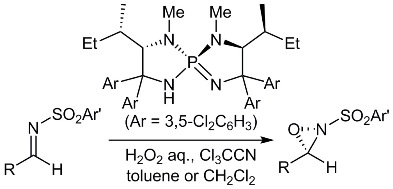
41.
Practical Preparation of Chiral N-Sulfonyl Oxaziridines by Catalytic Asymmetric Payne Oxidation
Ryosuke Tsutsumi, Seonwoo Kim, Daisuke Uraguchi, Takashi Ooi
Synthesis (PSP article) 2014, 46, 871-878
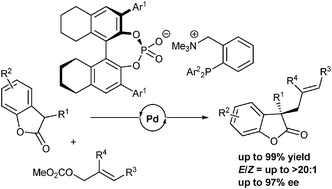
40.
Ligand-controlled E/Z selectivity and enantioselectivity in palladium-catalyzed allylation of benzofuranones with 1,2-disubstituted allylic carbonates
Kohsuke Ohmatsu, Mitsunori Ito , Takashi Ooi
Chem. Commun. 2014, 50, 4554-4557
39.
Asymmetric Alkylation of α-Cyanosulfones Catalyzed by Chiral 1,2,3-Triazolium Salts
Kohsuke Ohmatsu, Yusuke Hakamata, Ayano Goto, Takashi Ooi
Heterocycles 2014, 88, 1661-1666
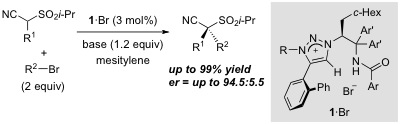
38.
Catalytic asymmetric Payne oxidation under the catalysis of P-spiro chiral triaminoiminophosphorane: Application to the synthesis of N-sulfonyl oxaziridines
Daisuke Uraguchi, Ryosuke Tsutsumi, Takashi Ooi
Tetrahedron 2014, 70, 1691-1701
DOI: 10.1016/j.tet.2013.12.086
Highlighted as a cover figure
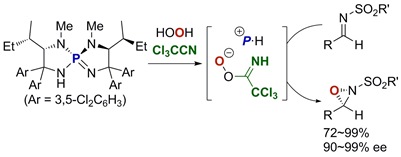
37.
Asymmetric Substitution at the Tetrasubstituted Chiral Carbon: Catalytic Ring-Opening Alkylation of Racemic 2,2-Disubstituted Aziridines with 3-Substituted Oxindoles
Kohsuke Ohmatsu, Yuichiro Ando , Takashi Ooi
J. Am. Chem. Soc. 2013, 135, 18706-18709
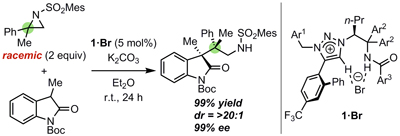
36.
Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocenters
Kohsuke Ohmatsu, Naomichi Imagawa, Takashi Ooi
Nature Chem. 2014, 6, 47-51
Highlighted in Synform 2014, A81-84. http://dx.doi.org/10.1055/s-0033-1339014 (Synstory)
Highlighted in Synfacts 2014, 354. http://dx.doi.org/10.1055/s-0033-1341001
Highlighted in Chem-Station (化学者のつぶやき)
中日新聞・日経産業新聞 (2013年11月25日)掲載
CBCテレビ(2013年11月25日)
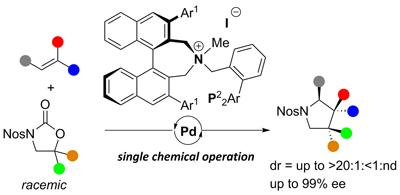
35.
Carbene Transfer from Triazolylidene Gold Complexes as a Potent Strategy for Inducing High Catalytic Activity
Daniel Canseco-Gonzalez, Ana Petronilho, Helge Mueller-Bunz, Kohsuke Ohmatsu, Takashi Ooi, Martin Albrecht
J. Am. Chem. Soc. 2013, 135, 13193-13203
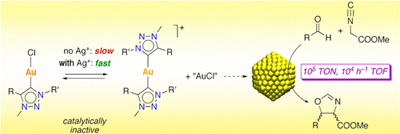
34.
Catalytic Asymmetric Oxidation of N-Sulfonyl Imines with Hydrogen Peroxide-Trichloroacetonitrile System
Daisuke Uraguchi, Ryosuke Tsutsumi, and Takashi Ooi
J. Am. Chem. Soc. 2013, 135, 8161-8164
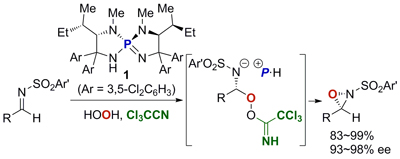
33.
Exploiting the Modularity of Ion-Paired Chiral Ligands for Palladium-Catalyzed Enantioselective Allylation of Benzofuran-2(3H)-ones
Kohsuke Ohmatsu, Mitsunori Ito, Tomoatsu Kunieda, and Takashi Ooi
J. Am. Chem. Soc. 2013, 135, 590-593
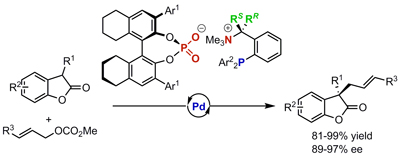
32.
Highly Stereoselective Michael Addition of Azlactones to Electron-Deficient Triple Bonds under P-Spiro Chiral Iminophosphorane Catalysis: Importance of Protonation Pathway
Uraguchi, Daisuke; Ueki, Yusuke; Sugiyama, Atsushi; Ooi, Takashi
Chem. Sci. 2013, 4, 1308-1311
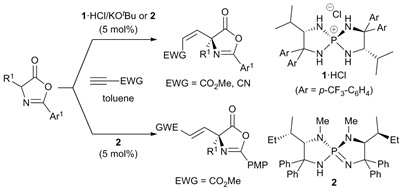
31.
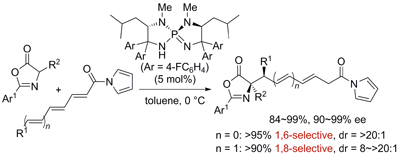
Highly Regio-, Diastereo-, and Enantioselective 1,6- and 1,8-Additions of Azlactones to Di- and Trienyl N-Acylpyrroles
Uraguchi, Daisuke; Yoshioka, Ken; Ueki, Yusuke; Ooi, Takashi
J. Am. Chem. Soc. 2012, 134, 19370-19373.
Highlighted in JACS Spotlights
Highlighted in Synfacts 2013, 217. http://dx.doi.org/10.1055/s-0032-1318035
30.
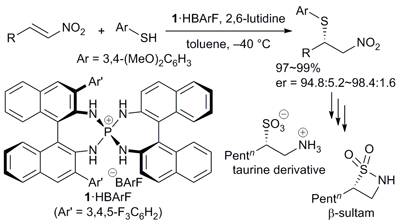
Chiral Ionic Brønsted Acid-Achiral Brønsted Base Synergistic Catalysis for Asymmetric Sulfa-Michael Addition to Nitroolefins
Uraguchi, Daisuke; Kinoshita, Natsuko; Nakashima, Daisuke; Ooi, Takashi
Chem. Sci. 2012, 3, 3161-3164.
29.
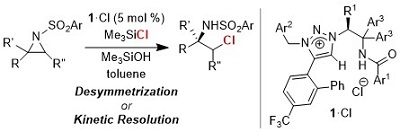
Catalytic Asymmetric Ring Openings of Meso and Terminal Aziridines with Halides Mediated by Chiral 1,2,3-Triazolium Silicates
Ohmatsu, Kohsuke; Hamajima, Yuta; Ooi, Takashi
J. Am. Chem. Soc. 2012, 134, 8794-8797.
Highlighted in Synfacts 2012, 892. http://dx.doi.org/10.1055/s-0032-1316631
Highlighted in Synfacts 2012, 911. http://dx.doi.org/10.1055/s-0032-1316697
28.
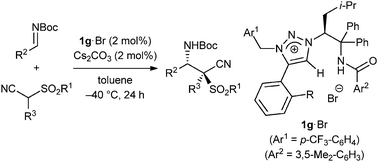
Catalytic Asymmetric Mannich-Type Reactions of α-Cyano α-Sulfonyl Carbanions
Ohmatsu, Kohsuke; Goto, Ayano; Ooi, Takashi
Chem. Commun. 2012, 48, 7913-7915.
27.
Nitroolefins as a Nucleophilic Component for Highly Stereoselective Aza-Henry Reaction under the Catalysis of Chiral Ammonium Betaines
Uraguchi, Daisuke; Oyaizu, Keigo; Ooi, Takashi
Chem. Eur. J. 2012, 18, 8306-8309.
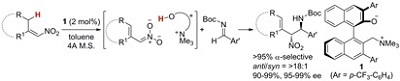
26.
Ionic Nucleophilic Catalysis of Chiral Ammonium Betaines for Highly Stereoselective Aldol Reaction from Oxindole-Derived Vinylic Carbonates
Uraguchi, Daisuke; Koshimoto, Kyohei; Ooi, Takashi
J. Am. Chem. Soc. 2012, 134, 6972-6975.
Highlighted in Synfacts 2012, 670. http://dx.doi.org/10.1055/s-0031-1291036
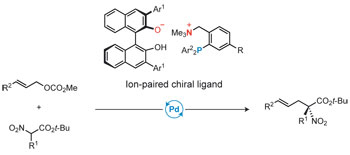
25.
Ion-paired chiral ligands for asymmetric palladium catalysis
Ohmatsu, Kohsuke; Ito, Mitsunori; Kunieda, Tomoatsu; Ooi, Takashi
Nat. Chem. 2012, 4, 473-477.
Highlighted in Nature Chemistry (News and Views)
Highlighted in Synfacts 2012, 876. http://dx.doi.org/10.1055/s-0032-1316660
Highlighted in Synform 2012, A113-A114. http://dx.doi.org/10.1055/s-0032-1316780 (Synstory)
中日新聞 (2012年4月2日朝刊)掲載
NHKおはよう東海(2012年4月2日)
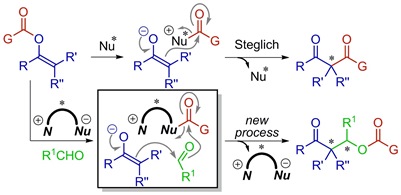
24.
Base-Catalyzed Direct Aldolization of α-Alkyl-α-Hydroxy Trialkyl Phosphonoacetates
Corbett, Michael; Uraguchi, Daisuke; Ooi, Takashi; Johnson, Jeffrey
Angew. Chem. Int. Ed. 2012, 51, 4685-4689.
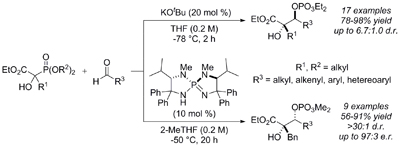
23.
Highly Stereoselective Catalytic Conjugate Addition of Acyl Anion Equivalent to Nitroolefins
Uraguchi, Daisuke; Ueki, Yusuke; Ooi, Takashi
Chem. Sci. 2012, 3, 842-845.
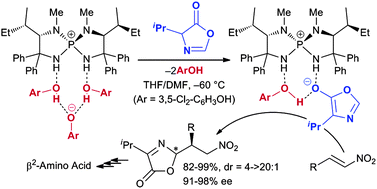
22.
Enantioselective Aza-Michael Addition to Conjugated Nitroenynes Catalyzed by Chiral Arylaminophosphonium Barfates
Uraguchi, Daisuke; Kinoshita, Natsuko; Kizu, Tomohito; Ooi, Takashi
Synlett. 2011, 1265-1267.
https://www.thieme-connect.com/ejournals/html/synlett/doi/10.1055/s-0030-1260541
SYNLETT Cluster: Brønsted Acid Catalysis
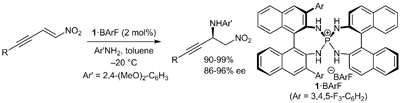
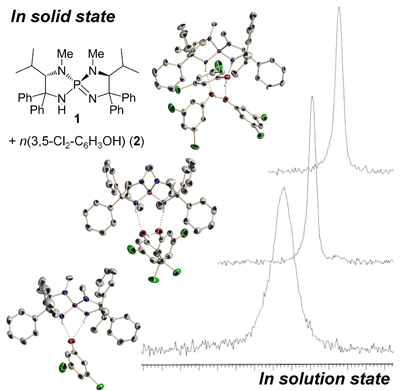
21.
Precise Control of Spontaneous Molecular Assembly of Chiral Tetraaminophosphonium Aryloxide-Arylhydroxide(s) in Solution
Uraguchi, Daisuke; Ueki, Yusuke; Ooi, Takashi
Angew. Chem. Int. Ed. 2011, 50, 3681-3683.
Selected as "Hot Paper"
20.
Chiral 1,2,3-Triazoliums as New Cationic Organic Catalysts with Anion-Recognition Ability: Application to Asymmetric Alkylation of Oxindoles
Ohmatsu, Kohsuke, Kiyokawa, Mari, Ooi, Takashi
J. Am. Chem. Soc. 2011, 133, 1307-1309.
Highlighted in Synfacts 2011, 324. http://dx.doi.org/10.1055/s-0030-1259452
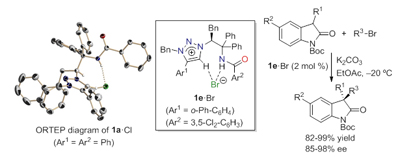
19.
Catalytic Asymmetric Protonation of α-Amino Acid-Derived Ketene Disilyl Acetals Using P-Spiro Diaminodioxaphosphonium Barfates as Chiral Proton
Uraguchi, Daisuke; Kinoshita, Natsuko; Ooi, Takashi
J. Am. Chem. Soc. 2010, 132, 12240-12242.
Highlighted in Synfacts 2010, 1300. http://dx.doi.org/10.1055/s-0030-1258790
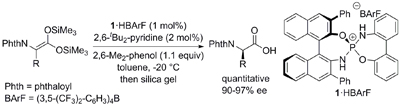
18.
Catalytic Asymmetric Direct Henry Reaction of Ynals: Short Syntheses of (2S,3R)-(+)-Xestoaminol C and (-)-Codonopsinines
Uraguchi, Daisuke; Nakamura, Shinji; Ooi, Takashi
Angew. Chem. Int. Ed. 2010, 49, 7562-7565.
Highlighted in Synfacts 2010, 1309. http://dx.doi.org/10.1055/s-0030-1258782
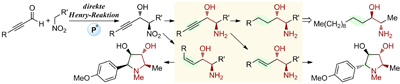
17.
Chiral Ammonium Betaines as an Ionic Nucleophilic Catalyst
Uraguchi, Daisuke; Koshimoto, Kyohei; Miyake, Shuhei; Ooi, Takashi
Angew. Chem. Int. Ed. 2010, 49, 5567-5569.
Highlighted in Synfacts 2010, 1075. http://dx.doi.org/10.1055/s-0030-1257971
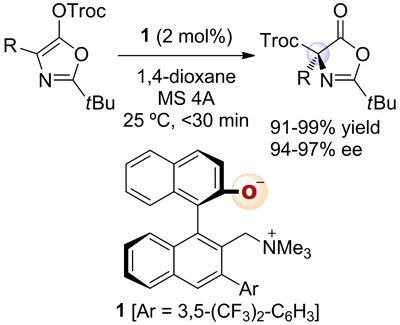
16.
Catalytic Asymmetric Hydrophosphonylation of Ynones
Uraguchi, Daisuke; Ito, Takaki; Nakamura, Shinji; Ooi, Takashi
Chem. Sci. 2010, 1, 488-490.
Highlighted in Synfacts 2011, 50. http://dx.doi.org/10.1055/s-0030-1259171
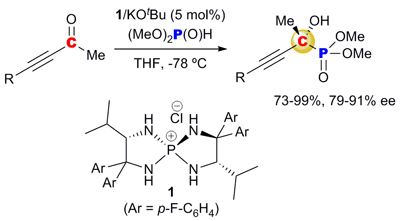
15.
Performance of C1-symmetric chiral ammonium betaines as a catalyst for the enantioselective Mannich-type reaction of α-nitrocarboxylates
Uraguchi, Daisuke; Koshimoto, Kyohei; Sanada, Chisato; Ooi, Takashi
Tetrahedron: Asymmetry. 2010, 21, 1189-1190.
14.
Flexible Synthesis, Structural Determination, and Synthetic Application of a New C1-Symmetric Chiral Ammonium Betaine
Uraguchi, Daisuke; Koshimoto, Kyohei; Ooi, Takashi
Chem. Commun. 2010, 46, 300-302.
http://www.rsc.org/Publishing/Journals/CC/article.asp?doi=b916627k
Selected as "Hot Article"
Highlighted in Synfacts 2010, 0232. http://dx.doi.org/10.1055/s-0029-1219139
13.
Diastereo- and Enantioselective Direct Henry Reaction of Pyruvates Mediated by Chiral P-Spiro Tetraaminophosphonium salts
Uraguchi, Daisuke; Ito, Takaki; Nakamura, Shinji; Sakaki, Sawako; Ooi, Takashi
Chem. Lett. 2009, 38, 1052.
http://www.jstage.jst.go.jp/article/cl/38/11/38_1052/_article
12.
Chiral Organic Ion Pair Catalysts Assembled Through a Hydrogen-Bonding Network
Uraguchi, Daisuke; Ueki, Yusuke; Ooi, Takashi
Science. 2009, 326, 120-123.
http://www.sciencemag.org/cgi/content/abstract/326/5949/120
Highlighted in C & EN 2009, 87, 25-26. [Science & Technology Concentrates (August 31)]
プレスリリース (名古屋大学HP)
読売新聞、日刊工業新聞 (8月28日朝刊)掲載
Highlighted in Synfacts 2009, 1408. http://dx.doi.org/10.1055/s-0029-1218214
11.
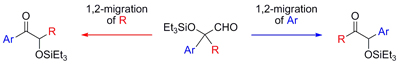
Al Lewis acid-catalyzed regiodivergent 1,2-rearrangement of α-siloxy aldehydes: scope and mechanism
Ohmatsu, Kohsuke; Tanaka, Takayuki; Ooi, Takashi; Maruoka, Keiji
Tetrahedron 2009, 65, 7516-7522.
10.
Chiral Arylaminophosphonium Barfates as a New Class of Charged Brønsted Acid for the Enantioselective Activation of Nonionic Lewis Bases
Uraguchi, Daisuke; Nakashima, Daisuke; Ooi, Takashi
J. Am. Chem. Soc. 2009, 131, 7242-7243.
http://pubs.acs.org/doi/abs/10.1021/ja903271t
Highlighted in Synfacts 2009, 562. http://dx.doi.org/10.1055/s-0029-1217582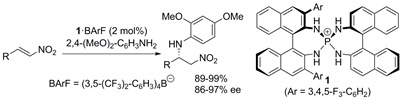
9.
Generation of Chiral Phosphonium Dialkyl Phosphite as a Highly Reactive P-Nucleophile: Application to Asymmetric Hydrophosphonylation of Aldehydes
Uraguchi, Daisuke; Ito, Takaki; Ooi, Takashi
J. Am. Chem. Soc. 2009, 131, 3836-3837.
http://pubs.acs.org/doi/abs/10.1021/ja810043d
Correction
J. Am. Chem. Soc. 2017, 139,3299?3299.
http://pubs.acs.org/doi/abs/10.1021/jacs.7b01263
Highlighted in Synfacts 2009, 562. http://dx.doi.org/10.1055/s-0029-1216583
Highlighted in Synfacts 2009, 645. http://dx.doi.org/10.1055/s-0029-1216717
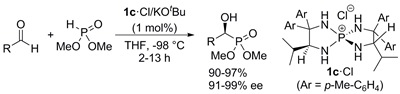
8.
Asymmetric Synthesis of α,α-Disubstituted-α-Amino Acids via Enantioselective Alkylation of Azlactones under Biphasic Conditions Using P-Spiro Chiral Tetraaminophosphonium Salts as a Phase-Transfer Catalyst
Uraguchi, Daisuke; Asai, Yoshihiro; Seto, Yuki; Ooi, Takashi
Synlett 2009, 658-660.
http://www.thieme-connect.com/ejournals/abstract/synlett/doi/10.1055/s-0028-1087812
SYNLETT Cluster: Phase Transfer Catalyst for Asymmetric Synthesis
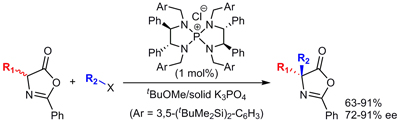
7.
Site-directed Asymmetric Quaternization of a Peptide Backbone at a C-Terminal Azlactone
Uraguchi, Daisuke; Asai, Yoshihiro; Ooi, Takashi
Angew. Chem. Int. Ed. 2009, 48, 733-737.
http://www.thieme-connect.com/ejournals/abstract/synlett/doi/10.1055/s-0028-1087812
Selected as a "inside cover picture"
Highlighted in Synfacts 2009, 205. http://dx.doi.org/10.1055/s-0028-1087586
6.
Chiral Tetraaminophosphonium Carboxylate-Catalyzed Direct Mannich-Type Reaction
Uraguchi, Daisuke; Ueki, Yusuke; Ooi, Takashi
J. Am. Chem. Soc. 2008, 130, 14088-14089.
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/asap/abs/ja806311e.html
Highlighted in Synfacts 2008, 1327. http://dx.doi.org/10.1055/s-0028-1083579
5.
Chiral Ammonium Betaines: A Bifunctional Organic Base Catalyst for Asymmetric Mannich-type Reaction of α-Nitrocarboxylates
Uraguchi, Daisuke; Koshimoto, Kyohei; Ooi, Takashi
J. Am. Chem. Soc. 2008, 130, 10878-10879.
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/asap/abs/ja8041004.html
Highlighted in Synfacts 2008, 1105. http://dx.doi.org/10.1055/s-2008-1078149 (Synfact of the month)
4.
Synthesis of Chiral Tetraaminophosphonium Chlorides from N-BOC α-Amino Acid Esters
Uraguchi, Daisuke; Sakaki, Sawako; Ueki, Yusuke; Ito, Takaki; Ooi, Takashi
Heterocycles 2008, 76, 1081-1085.
http://www.heterocycles.jp/library/abstract.php?article=3158
3.
Chiral Tetraaminophosphonium Salt-Mediated Asymmetric Direct Henry Reaction
Uraguchi, Daisuke; Sakaki, Sawako; Ooi, Takashi
J. Am. Chem. Soc. 2007, 129, 12392-12393.
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/2007/129/i41/abs/ja075152+.html
Highlighted in Synfacts 2007, 1309. http://dx.doi.org/10.1055/s-2007-991331 (Synfact of the month)
Highlighted in Synform 2007, A7-A8. http://dx.doi.org/10.1055/s-2007-1000843 (Synstory)
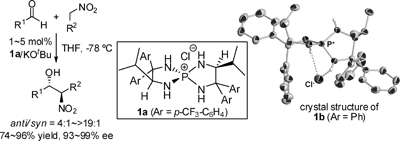
2.
Advantage of in situ generation of N-arylsulfonyl imines from α-amide sulfones in the phase-transfer-catalyzed asymmetric Strecker reaction
Ooi, Takashi; Uematsu, Yukitaka; Fujimoto, Jun; Fukumoto, Kazuhiro; Maruoka, Keiji
Tetrahedron Letters 2007, 48, 1337-1340.
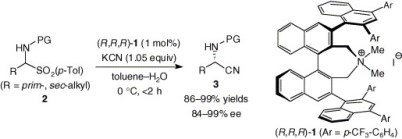
1.
Asymmetric Strecker Reaction of Aldimines Using Aqueous Potassium Cyanide by Phase-Transfer Catalysis of Chiral Quaternary Ammonium Salts with a Tetranaphthyl Backbone.
Ooi, Takashi; Uematsu, Yukitaka; Maruoka, Keiji
J. Am. Chem. Soc 2006, 128, 2548-2549.
総説等 : Reviews
-
12.
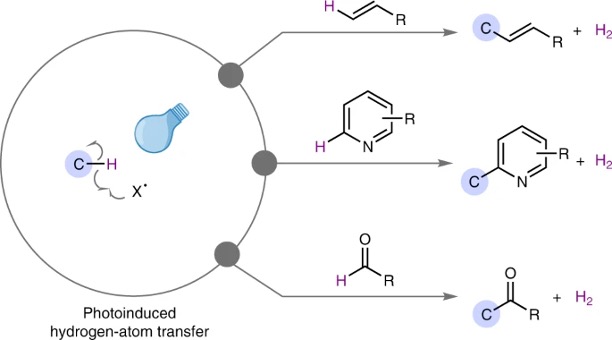
Catalytic acceptorless dehydrogenative coupling mediated by photoinduced hydrogen-atom transfer
Kohsuke Ohmatsu, Takashi Ooi
Nature Synth. 2023, xx, xxxx-xxxx.
-
11.
Catalysis of chiral iminophosphorane for simultaneous control of multiple selectivity: experimental and theoretical investigation
浦口 大輔, 大井 貴史
有機合成化学協会誌 2021, 79, 406-416.
-
10.
Cationic Organic Catalysts or Ligands in Concert with Metal
Kohsuke Ohmatsu, Takashi Ooi
Topics in Current Chemistry 2019, 377, 31.
-
9.
Chemistry of Ammonium Betaines: Application to Ion-Pair Catalysis for Selective Organic Transformations
浦口 大輔, 大井 貴史
有機合成化学協会誌 2018, 76, 1144-1153.

-
8.
Design of supramolecular chiral ligands for asymmetric metal catalysis
Kohsuke Ohmatsu, Takashi Ooi
Tetrahedron Letters 2015, 56, 2043-2048.
-
7.
Development of Ion-Paired Chiral Ligands for Asymmetric Transition-Metal Catalysis
大松 亨介, 大井 貴史
有機合成化学協会誌 2015, 73, 140-150.
-
6.
Development of P-Spiro Chiral Aminophosphonium Salts as a New Class of Versatile Organic Molecular Catalyst
浦口 大輔, 大井 貴史
有機合成化学協会誌 2010, 68, 1185-1194.
http://www.jstage.jst.go.jp/article/yukigoseikyokaishi/68/11/1185/_pdf/-char/ja/
-
5.
「水素結合供与型キラルホスホニウム/有機アニオンが示す新たなイオン「対」触媒作用」
上木 佑介, 浦口 大輔, 大井 貴史
触媒 2010, 52, 509-514.
-
4.
「P-スピロ型テトラアミノホスホニウム塩の創製と有機分子触媒としての機能発現」
浦口 大輔
触媒 2008, 50, 683-687.
-
3.
Development and Applications of C2-Symmetric, Chiral, Phase-Transfer Catalysts
Ooi, Takashi; Maruoka, Keiji
Aldrichimia Acta 2007, 40, 77-86.
-
2.
Design of Chiral Organocatalysts for Practical Asymmetric Synthesis of Amino Acid Derivatives
Maruoka, Keiji; Ooi, Takashi; Kano, Taichi
Chem. Commun. 2007, 1487-1495.
-
1.
Recent Advances in Asymmetric Phase-Transfer Catalysis
Ooi, Takashi; Maruoka, Keiji
Chem. Commun. 2007, 46, 4222-4266.
解説・紀要等
-
10.
「人工ストリゴラクトンの設計」
土屋 雄一朗, 浦口 大輔, 大井 貴史
植物の生長調節 2019, 54(1), 49-53.
-
9.
「有機イオン対の触媒化学:構造に由来する機能発現」
浦口 大輔, 大松 亨介, 大井 貴史
ケミカルタイムズ 2018, (2), 20-26.
-
8.
「有機分子触媒の化学-「輪」をもって尊しとなす-」
浦口 大輔, 大井 貴史
現代化学 2017, 558(9), 52-56.
-
7.
α-アミノカルボニル化合物の効率的不斉合成
中島 翼,大松 亨介, 大井 貴史
PHARMSTAGE 2017, 17, 61-67.
-
6.
「[5.5]-P-スピロ型キラルテトラアミノホスホニウム塩を用いる触媒的分子変換」
浦口 大輔, 大井 貴史
Wako Organic Square 2017, 59(3), 2-5.
-
5.
「Lectureship Award MBLA 2014受賞講演ツアーを終えて」
浦口 大輔
有機合成化学協会誌 2015, 73, 653-662.
-
4.
「忘れられた反応剤Davis’オキサジリジン」
浦口 大輔, 堤 亮祐, 大井 貴史
月刊「化学」 2013, 68, 66-67.
-
3.
「D2対称型光学活性テトラアミノホスホニウム塩の創製と相間移動触媒反応への応用」
浦口 大輔, 大井 貴史
月刊ファインケミカル 2010, 39, 42-48.
-
2.
「光学活性な炭素の三角形を作る―Corey-Chaykovsky反応と親電子剤活性化法の組み合わせ」
浦口 大輔, 大井 貴史
化学 2008, 63, 66-67.
-
1.
「二相系でのイミンの系内発生を活かした触媒的不斉合成」
大井 貴史
Organometallic News 2008, 40-43
著書等 : Books
-
14.
“Organic Molecular Catalysts in Radical Chemistry: Challenges Toward Selective Transformations”
Daisuke Uraguchi, Kohsuke Ohmatsu, Takashi Ooi
“Molecular Technology: Synthesis Innovation, Volume 4” H. Yamamoto and T. Kato Eds. Wiley-VCH; 163-197 (2019) -
13.
「多機能型キラルオニウム塩の設計に基づく高選択的分子変換」
大松 亨介, 浦口 大輔, 大井 貴史
“有機分子触媒の開発と工業利用” シーエムシー出版; xxx-xxx (2018) -
12.
「イオン対を中心とした不斉塩基触媒」
浦口 大輔, 大井 貴史
“CSJカレントレビュー「有機分子触媒の化学」” 日本化学会; 109-117 (2016) -
11.
“Site-Selective Conjugate Addition through Catalytic Generation of Ion-Pairing Intermediates”
Daisuke Uraguchi, Takashi Ooi
“Topics in Current Chemistry: Site Selective Catalysis” T. Kawabata Ed. Springer; 55-84 (2016) -
10.
「イオン性キラル触媒~新しい分子設計へのこだわり~」
大松 亨介, 大井 貴史
“CSJカレントレビュー「キラル化学:その起源から最新のキラル材料研究まで」” 八島 栄治 日本化学会; (2013) -
9.
“Axially Chiral C2-Symmetric Catalysts”
Daisuke Uraguchi, Kohsuke Ohmatsu, Takashi Ooi
“Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications” P. I. Dalko Eds. Wiley-VCH; 161-193 (2013) -
8.
“Asymmetric phase-transfer catalysis”
Kohsuke Ohmatsu, Daisuke Uraguchi, Takashi Ooi
“Stereoselective Synthesis of Drugs and Natural Products” V. Andrushko, N. Andrushko Eds. Wiley-VCH; 119-144 (2013) -
7.
"6.1 C-C Bond Formation: Alkylation"
Uraguchi, Daisuke; Ooi, Takashi
"COMPREHENSIVE CHIRALITY" Elsevier Science; 1-36 (2012)
-
6.
"Hydrogen-Bonding Catalysts Other than Ureas and Thioureas"
Uraguchi, Daisuke; Ooi, Takashi
"Science of Synthesis: Asymmetric Organicatalysis 2" Thieme; 413-435 (2012)
-
5.
"Development of phase-transfer-catalyzed asymmetric Strecker reaction based on the molecular design of chiral quaternary ammonium salts"
Ooi, Takashi
"ACS Symposium Series (2009), 1009(Asymmetric Synthesis and Application of .alpha.-Amino Acids)" ACS Publications; 177-189 (2009)
-
4.
“Chiral quaternary ammonium fluorides for asymmetric synthesis”
Shirakawa, Seiji; Ooi, Takashi; Maruoka, Keiji
“Asymmetric Phase Transfer Catalysis” K. Maruoka Eds. Wiley-VCH; 189-206 (2008) -
3.
“Cinchona-derived chiral phase-transfer catalysts for amino acid synthesis”
T. Ooi
“Asymmetric Phase Transfer Catalysis” K. Maruoka Eds. Wiley-VCH; 9-33 (2008) -
2.
“Chiral Carbonyl Lewis Acid Complexes in Asymmetric Syntheses”
K. Maruoka, T. Ooi
“Asymmetric Synthesis - The Essentials” M. Christmann, S. Bräse Eds. VCH; 121-125 (2007) -
1.
“Ammonium Ions as Chiral Templates”
T. Ooi, K. Maruoka
“Enantioselective Organocatalysis: Reactions and Experimental Procedures” P. I. Dalko Ed. Wiley; 121-150 (2007)



