Member忍久保研の構成員
准教授:福井 識人
Dr. Norihito Fukui

略歴
| 2013年3月 | 京都大学 理学部 卒業 |
|---|---|
| 2015年3月 | 京都大学大学院 理学研究科修士課程 修了 (指導教官:大須賀 篤弘 教授、依光 英樹 准教授(当時)) |
| 2015年4月–2018年3月 | 日本学術振興会特別研究員(DC1) |
| 2018年3月 | 京都大学大学院 理学研究科 博士後期課程 修了 (指導教官:大須賀 篤弘 教授) 博士(理学)取得 |
| 2018年4月–2022年3月 | 名古屋大学大学院 工学研究科 助教 |
| 2021年10月–2025年3月 | JSTさきがけ研究者「物質探索空間の拡大による未来材料の創製」領域1期生 |
| 2022年4月–2025年3月 | 名古屋大学大学院 工学研究科 講師 |
| 2025年4月– | 名古屋大学大学院 工学研究科 准教授 |
| 2025年4月– | JST創発的支援事業 創発研究者(グンパネル) |
業績
| CV | |
|---|---|
| ORCiD | 0000-0002-0466-0116 |
| ResearcherID | N-6736-2018 |
| researchmap | 70823277 |
専門分野
有機化学、構造有機化学
原著論文
71. Effect of structural bending on the photophysical properties of perylene bisimide
H. Sotome,* M. Higashi,* Y. Tanaka, H. Shinokubo, Y. Kobori, N. Fukui*
J. Chem. Phys., 2025, 162, 114305.
70. Captodative Approach to Stable Nitrogen-Centered Radicals, Anions, and Cations Exhibiting Near-Infrared Electrochromism
K. Tajima, C. Bucher,* D. Shimizu, N. Fukui,* H. Shinokubo*
JACS Au, 2025 .
69. Synthesis and Properties of Donor–Acceptor-Type Cyclobisbiphenylenecarbonyls
E. Nishimoto, T. Ikai, H. Shinokubo, N. Fukui*
Chem. Eur. J. 2024 , e202404194.
68. Synthesis and electron-transporting properties of phenazine bisimides
K. Tajima, T. Moribe, K. Matsuo, H. Yamada,* S. Seki,* S. Yokokura, T. Shimada, N. Fukui,* H. Shinokubo*
J. Mater. Chem. C, 2025, 13, 655-662.
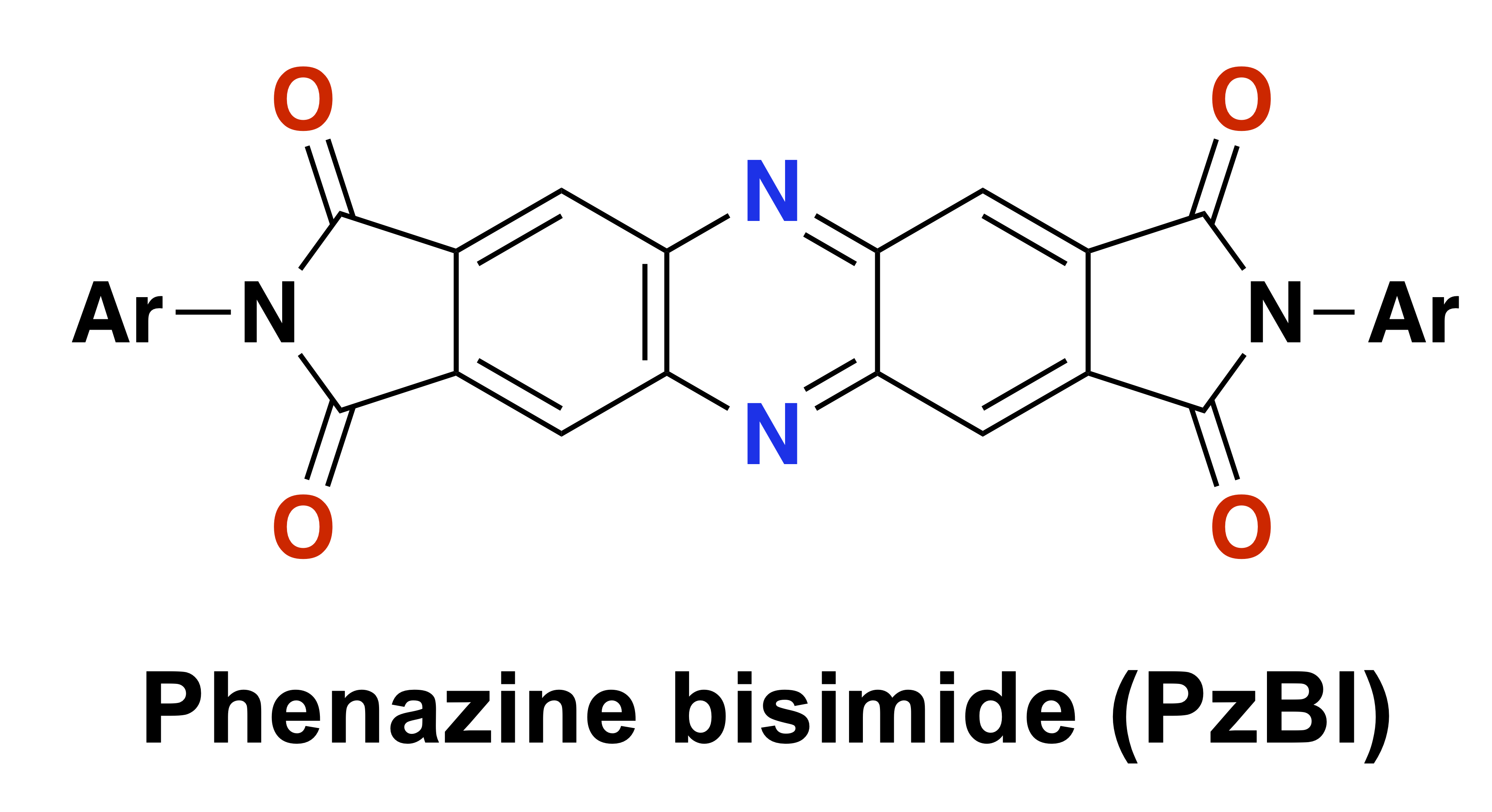
67. Inner-Bond-Cleavage Approach to Figure-Eight Macrocycles from Planar Aromatic Hydrocarbons
R. Yoshina, J. Hirano, E. Nishimoto, Y. Sakamoto, K. Tajima, S. Minabe, M. Uyanik, K. Ishihara, T. Ikai, E. Yashima, T. Omine, F. Ishiwari, A. Saeki, J. Kim, J. Oh, D. Kim, G. Liu, T. Yasuda, H. Shinokubo, N. Fukui*
J. Am. Chem. Soc. 2024.
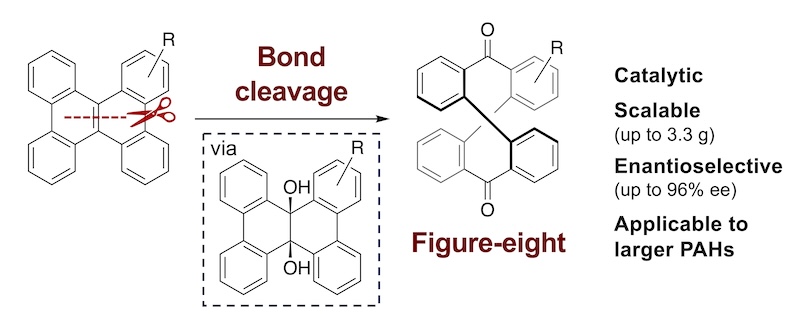
66. End-to-End Bent Perylene Bisimide Cyclophanes by Double Sulfur Extrusion
Y. Tanaka, K. Tajima, R. Kusumoto, Y. Kobori,* N. Fukui,* H. Shinokubo*
J. Am. Chem. Soc. 2024, 146, 16332–16339.
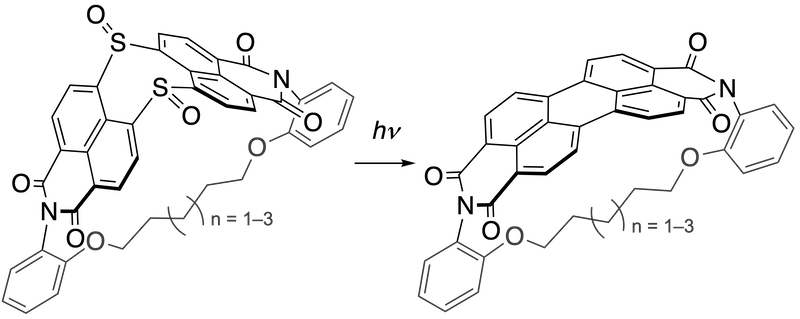
65. Homochiral and Heterochiral Self-Sorting Assemblies of Antiaromatic Ni(II) Norcorrole Dimers
S. Y. Liu, S. Li, S. Ukai, R. Nozawa, N. Fukui, R. Sugimori, R. Kishi, H. Shinokubo*
Chem. Eur. J. 2024, e202400292.
64. Changing aromatic properties through stacking: the face-to-face dimer of Ni(II) bis(pentafluorophenyl)norcorrole
Q. Wang, D. Sundholm,* J. Gauss, T. Nottoli, F. Lipparini, S. Kino, S. Ukai, N. Fukui, H. Shinokubo.
Phys. Chem. Chem. Phys. 2024, 26, 14777-14786.
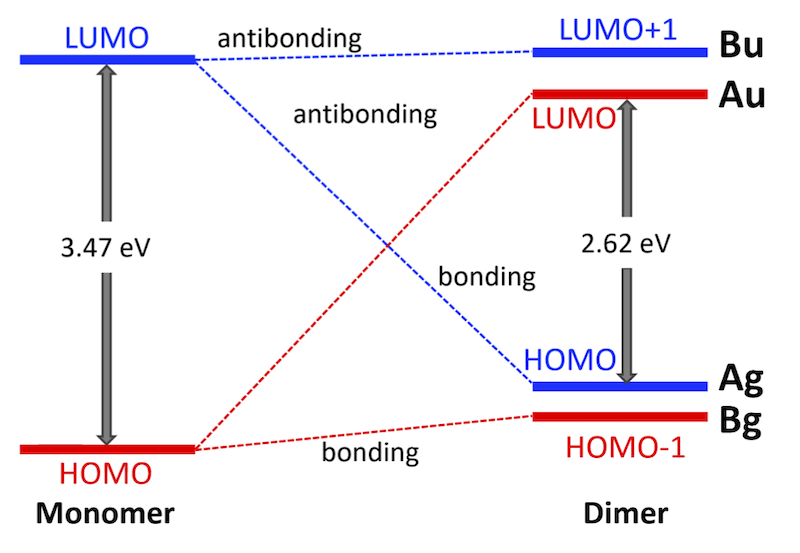
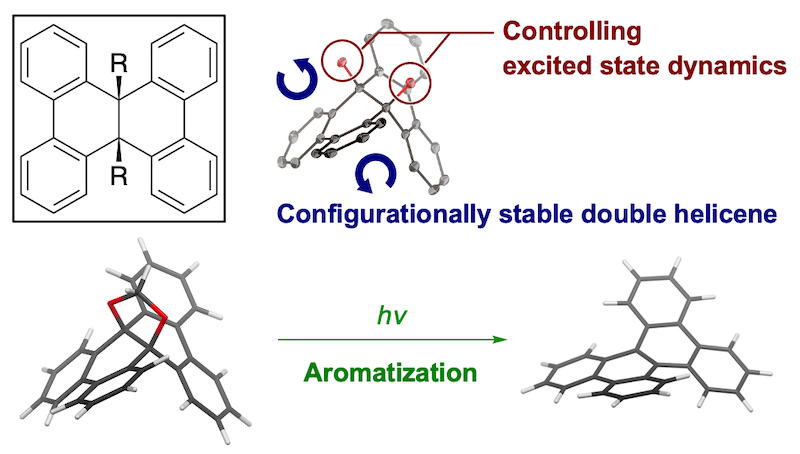
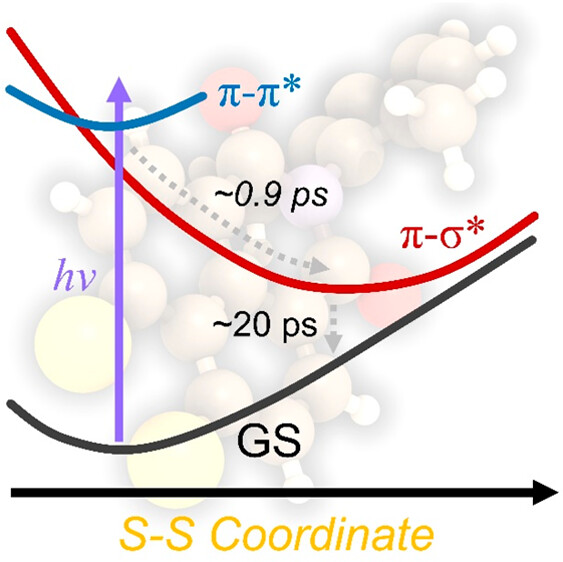
63. Synthesis of sterically congested double helicene by alkyne cycloisomerization
J. Hirano, S. Miyoshi, E. Yashima, T. Ikai, H. Shinokubo, N. Fukui,*
Chem. Commun. 2024, 60, 6035-6038.
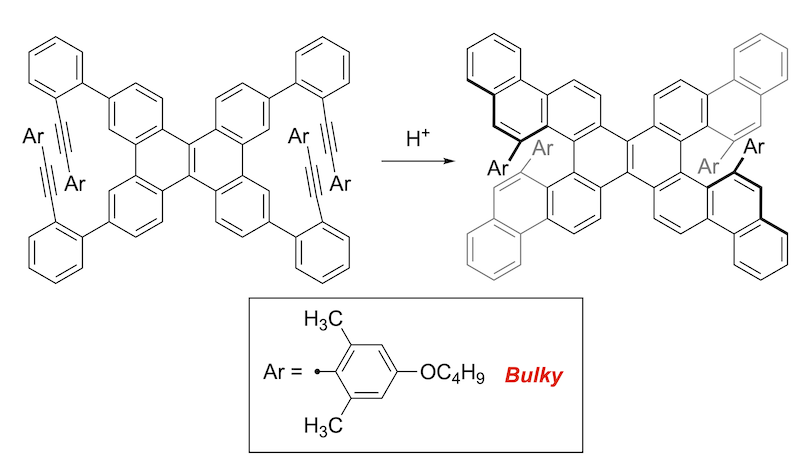
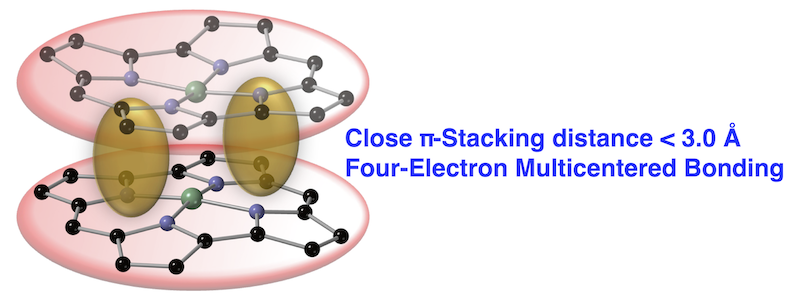
62. Close Stacking of Antiaromatic Ni(II) Norcorrole Originating from a Four-Electron Multicentered Bonding Interaction
S. Kino, S. Ukai, N. Fukui, R. Haruki, R. Kumai, Q. Wang, S. Horike, Q. M. Phung, D. Sundholm,* H. Shinokubo*
J. Am. Chem. Soc. 2024, 146, 9311-9317.
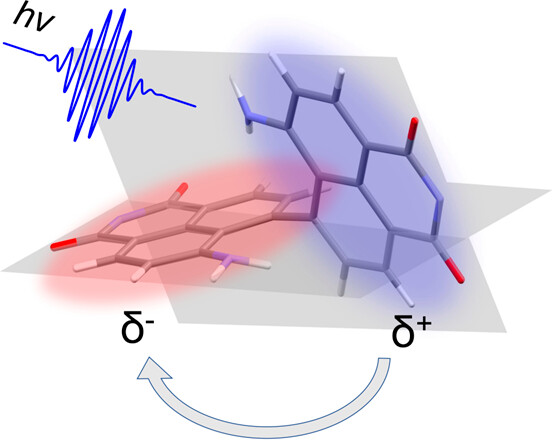
61. Excimer Formation Driven by Excited-State Structural Relaxation in a Covalent Aminonaphthalimide Dimer
R. Jing, Y. Li, K. Tajima, Y. Wan, N. Fukui,* H. Shinokubo,* Z. Kuang,* A. Xia*
J. Phys. Chem. Lett. 2024, 15, 1469-1476.
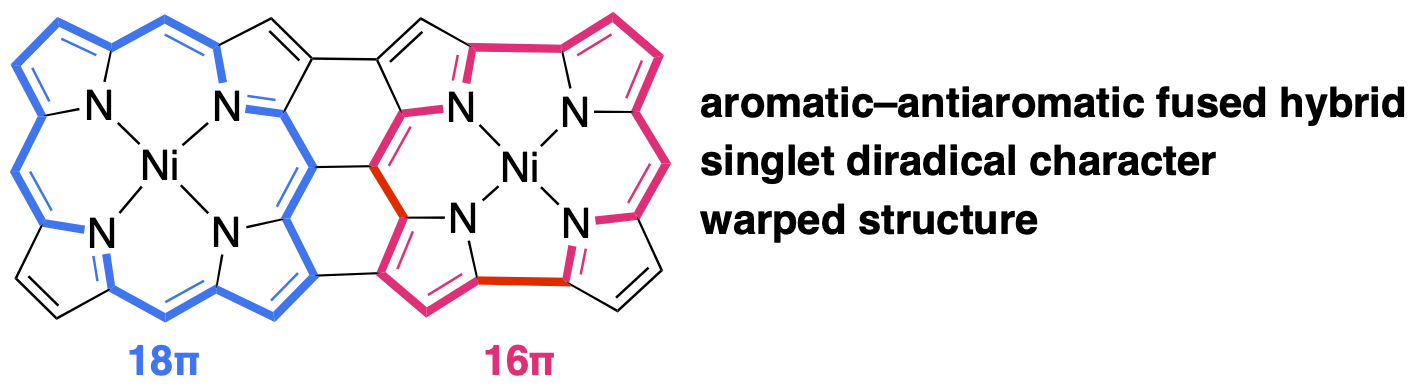
60. A Triply Linked Porphyrin−Norcorrole Hybrid with Singlet Diradical Character
K. Wang, S. Ito, S. Ren, D. Shimizu, N. Fukui, R. Kishi, Q. Liu,* A. Osuka, J. Song,* H. Shinokubo*
Angew. Chem. Int. Ed. 2024, e202401233.
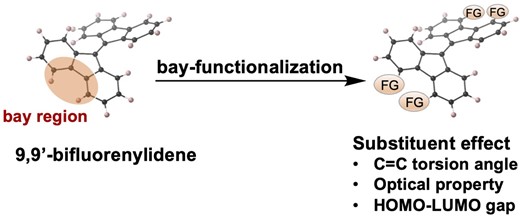
59. Synthesis and properties of bay-functionalized 9,9′-bifluorenylidene derivatives
J. Hirano, H. Shinokubo, N. Fukui*
Chem. Lett. 2024, 53upad015.
58. Effect of Internal Substituents on the Properties of Dibenzo[g,p]chrysene
Y. Takeo, J. Hirano, N. Fukui,* H. Shinokubo*
Org. Lett. 2023, 25, 8484–8488.
57. Effect of internal oxygen substituents on the properties of bowl-shaped aromatic hydrocarbons
Y. Takeo, J. Hirano, D. Shimizu, N. Fukui,* H. Shinokubo*
Org. Chem. Front. 2023, 10, 5895–5901.

56. Intrinsic Photostability in Dithiolonaphthalimide Achieved by Disulfide Bond-Induced Excited-State Quenching
Z. Wang, R. Jing, Y. Li, D. Song, Y. Wan, N. Fukui,* H. Shinokubo,* Z. Kuang,* and A. Xia*
J. Phys. Chem. Lett. 2023, 14, 8485–8492.
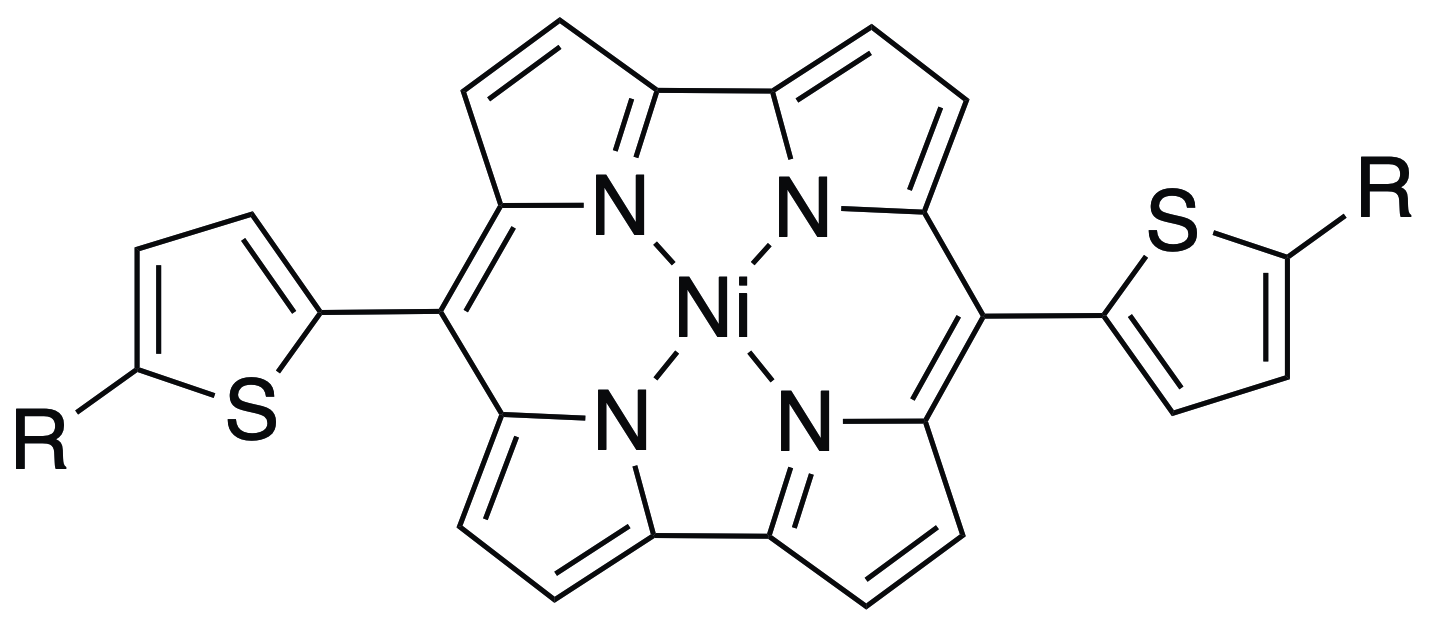
55. Highly soluble Ni(II) dithienylnorcorrole
S. Itabuchi, R. Nozawa, T. Yoshida, N. Fukui, H. Shinokubo*
J. Porphyrins Phthalocyanines. 2023, 27, 121-125.
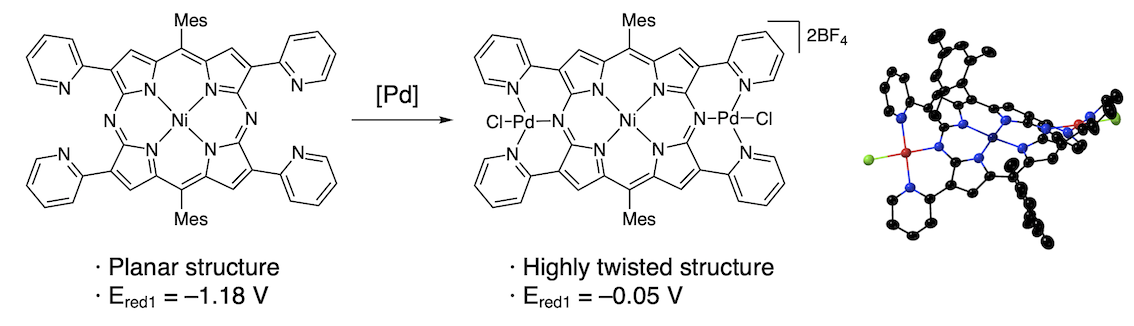
54. Easily Switchable 18π-, 19π-, and 20π-Conjugation of Diazaporphyrin Double-Pincer Bispalladium Complexes.
T. Sakurai, Y. Hiraoka, H. Tanaka, Y. Miyake, N. Fukui, H. Shinokubo*
Angew. Chem. Int. Ed. 2023, in press.
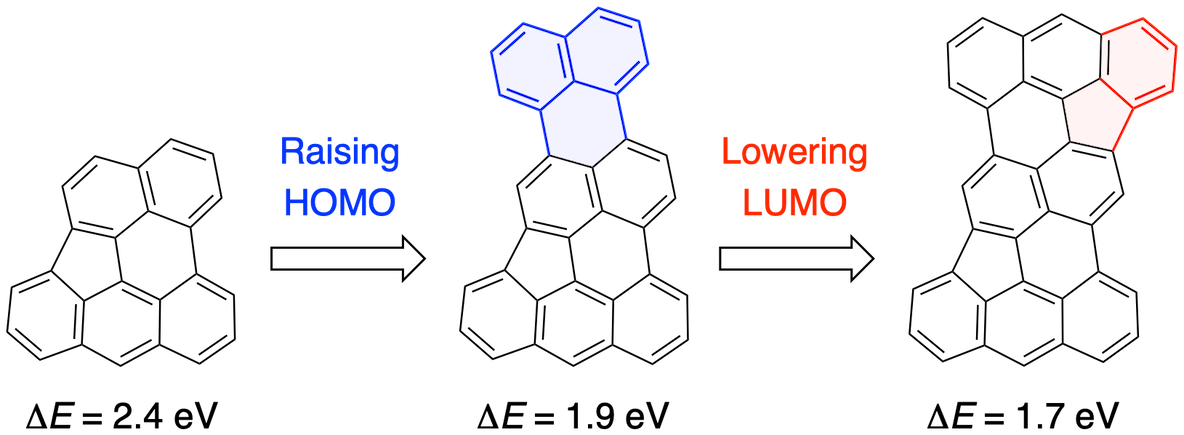
53. Near-Infrared-Responsive Hydrocarbons Designed by π-Extension of Indeno[1,2,3,4-pgra]perylene at the 1,2,12-Positions.
M. Kato, J. Kim, J. Oh, D. Shimizu, N. Fukui,* H. Shinokubo*
Chem. Eur. J. 2023, in press.
52. One-dimensional stacking array of 10,20-diphenyl-5,15-diazaporphyrin metal complexes.
S. Mori, T. Sakurai, T. Nishimura, N. Fukui, Y. Miyake,* H. Shinokubo*
J. Porphyrins Phthalocyanines. 2023, in press.
51. Dinaphthooxepine Bisimide Undergoes Oxygen Extrusion Reaction upon Electron Injection at Room Temperature
M. Odajima, N. Fukui,* H. Shinokubo*
Org. Lett. 2023, 25, 282–287.
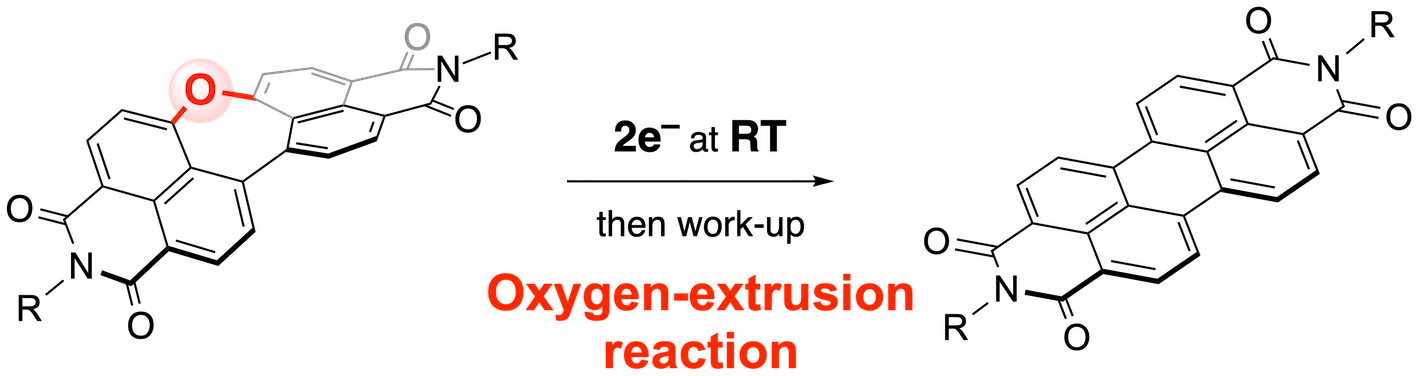
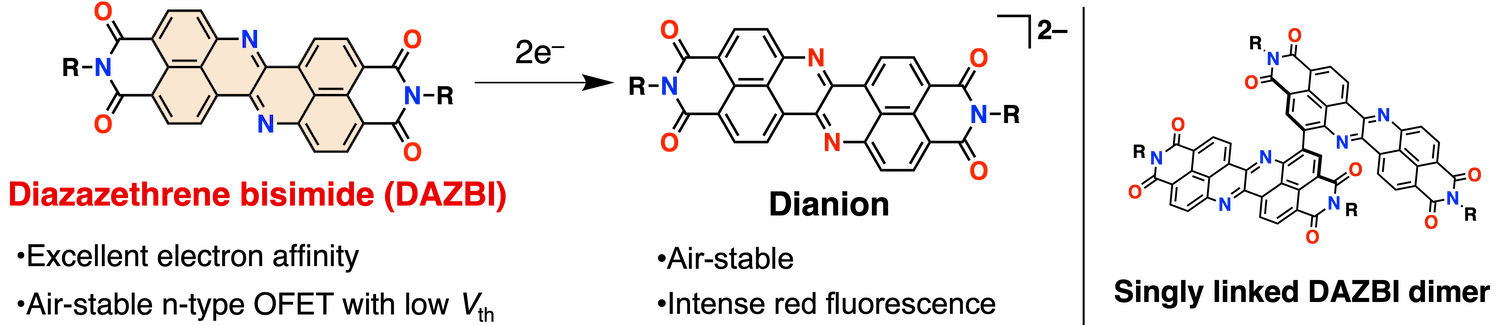
50. Diazazethrene Bisimide: A Strongly Electron-Accepting π-System Synthesized via the Incorporation of both Imide Substituents and Imine-type Nitrogen Atoms into Zethrene
K. Tajima, K. Matsuo, H. Yamada, N. Fukui,* H. Shinokubo*
Chem. Sci. 2023, 14, 635-642.
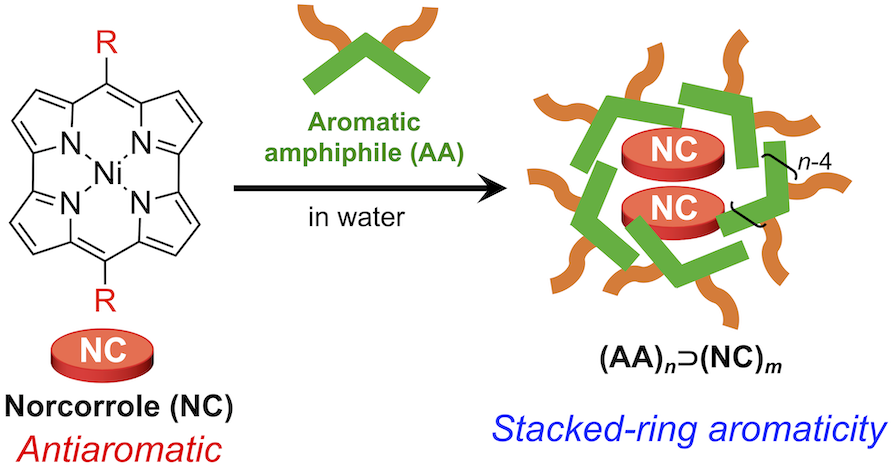
49. Realization of Stacked-Ring Aromaticity in a Water-Soluble Micellar Capsule
S. Liu, N. Kishida, J. Kim, N. Fukui, R. Haruki, Y. Niwa, R. Kumai, D. Kim,* M. Yoshizawa,* H. Shinokubo*
J. Am. Chem. Soc. 2023, 145, 2135-2141.
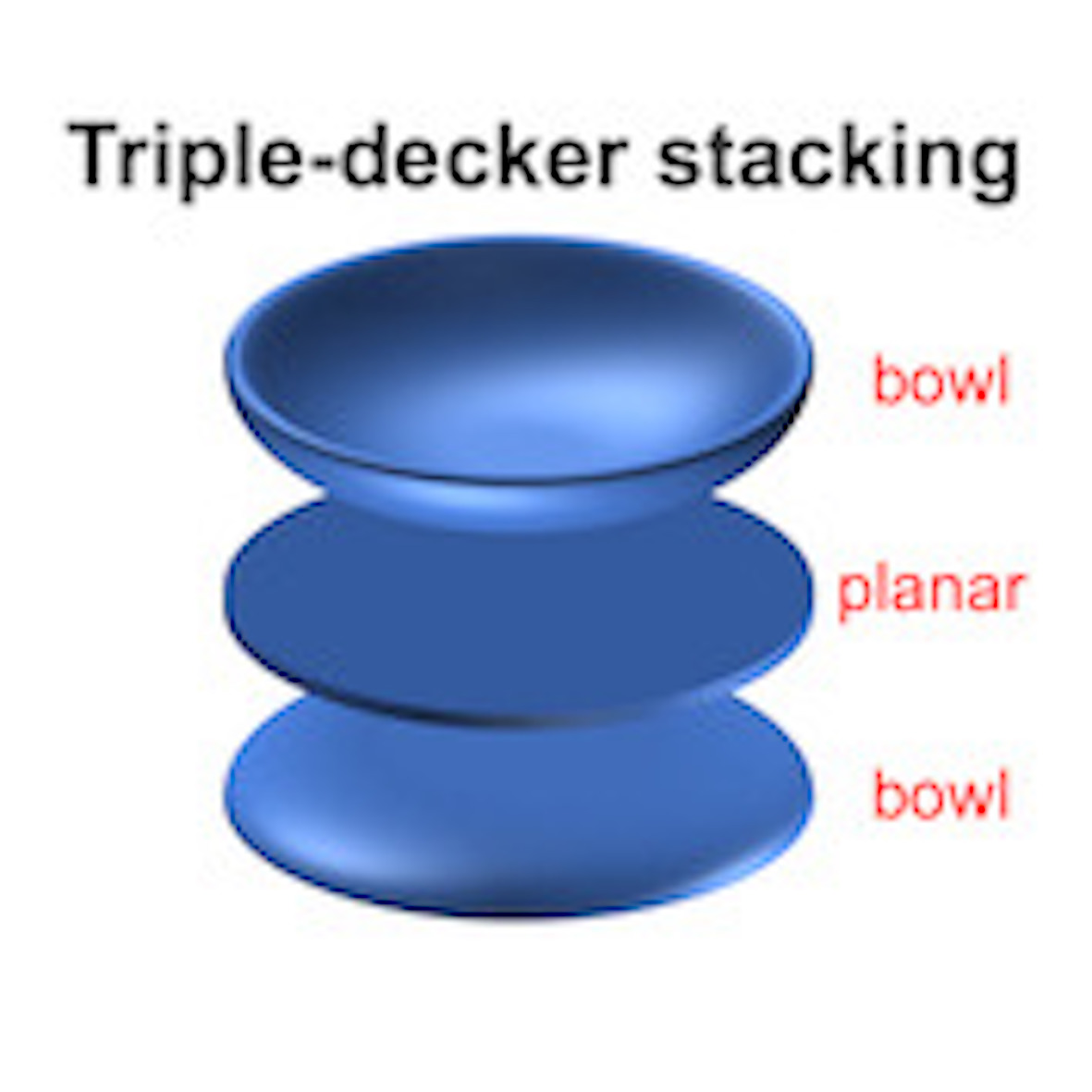
48. Planarization of a bowl-shaped molecule by triple-decker stacking
H. Kawashima, N. Fukui, Q. M. Phung,* T. Yanai, H. Shinokubo*
Cell. Rep. Phys. Sci. 2022, 3, 101045
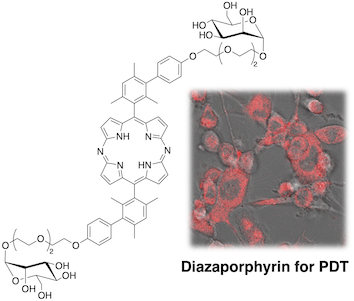
47. D-Mannose-appended 5,15-diazaporphyrin for photodynamic therapy
L. M. A. Ali, K. Miyagawa, N. Fukui, M. Onofre, K. Elcheikh, A. Morère, S. Clément, M. Gary-Bobo,* S. Richeter,* H. Shinokubo*
Org. Biomol. Chem. 2022, 20, 8217-8222
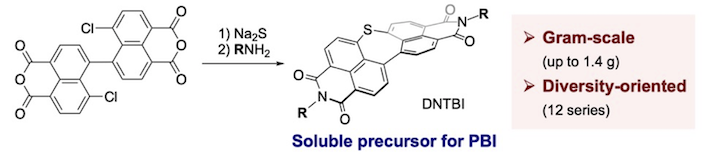
46. Gram-Scale Diversity-Oriented Synthesis of Dinaphthothiepine Bisimides as Soluble Precursors for Perylene Bisimides
Y. Tanaka, K. Matsuo,* H. Yamada,* N. Fukui,* H. Shinokubo*
Eur. J. Org. Chem. 2022, 31, e202200770.
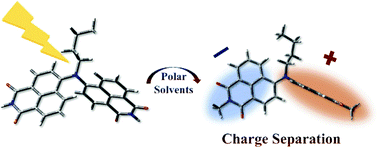
45. Symmetry-breaking charge separation in a nitrogen-bridged naphthalene monoimide dimer
X. Niu, K. Tajima, J. Kong, M. Tao, N. Fukui,* Z. Kuang,* H. Shinokubo,* A.Xia*
Phys. Chem. Chem. Phys. 2022, 24, 14007-14015
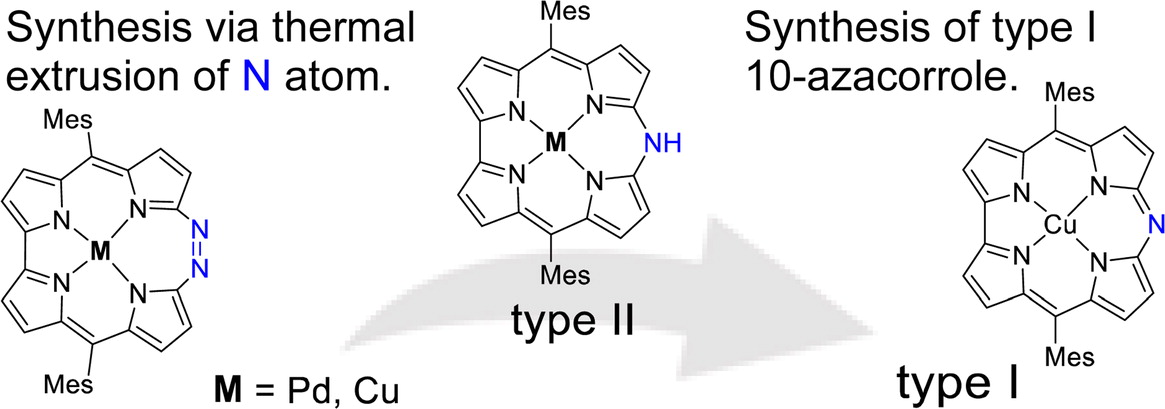
44. Nitrogen Extrusion of Diazacorrphycenes to Azacorroles and Synthesis of Two Types of Copper 10-Azacorrole Complexes
A.Yagi, N. Okada, N. Fukui, H. Tanaka, T. Hatakeyama, H. Shinokubo*
Chem. Lett. 2022, 51, 321-324
43. Synthesis of Dibenzo[h,t]rubicene through Its Internally Dimethoxy-Substituted Precursor
M. Kato, N. Fukui,* H. Shinokubo*
Chem. Lett. 2022, 51, 288-291
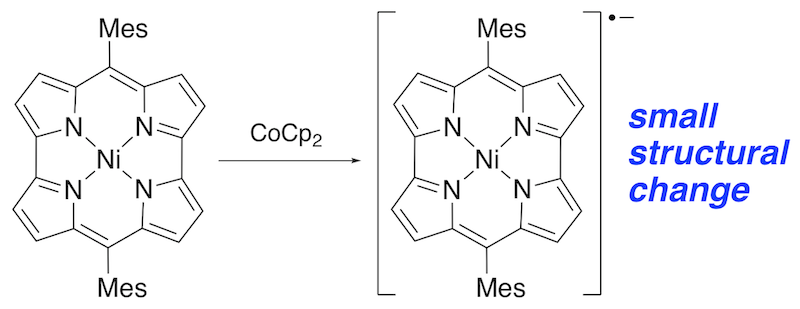
42. Isolation and Structure Analysis of a Ni(II) Norcorrole Radical Anion
S. Ukai, N. Fukui, T. Ikeue, H. Shinokubo*
Chem. Lett. 2022, 51, 181-184
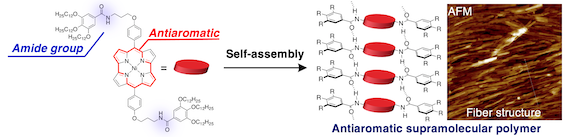
41. A Supramolecular Polymer Constituted of Antiaromatic Ni(II) Norcorroles
S. Ukai, A. Takamatsu, M. Nobuoka, Y. Tsutsui, N. Fukui, S. Ogi,* S. Seki,* S. Yamaguchi,* H, Shinokubo*
Angew. Chem. Int. Ed. 2022, 61, e202114230.
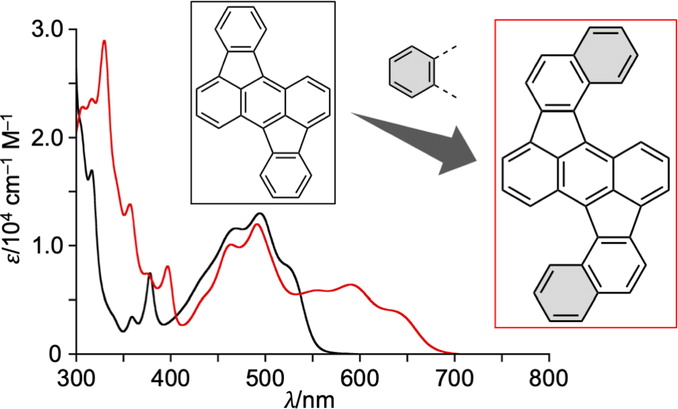
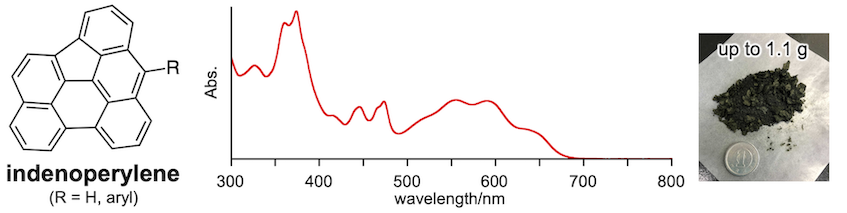
40. Indeno[1,2,3,4-pqra]perylene: A Medium-Sized Aromatic Hydrocarbon Exhibiting Full-Range Visible-Light Absorption
M. Kato, N. Fukui,* H, Shinokubo*
Chem. Eur. J. 2022, 28, e202103647.
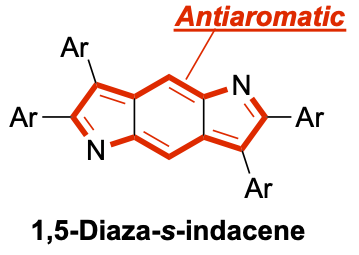
39. Antiaromatic 1,5-Diaza-s-indacenes
K. Hanida, J. Kim, N. Fukui, Y. Tsutsui, S. Seki,* D. Kim,* H. Shinokubo*
Angew. Chem. Int. Ed. 2021, 20765-20770.
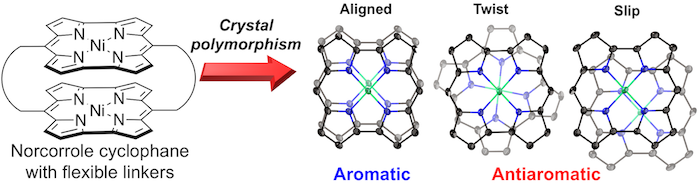
38. Determinant Factors of Three-Dimensional Aromaticity in Antiaromatic Cyclophanes
H. Kawashima, S. Ukai, R. Nozawa, N. Fukui, G. Fitzsimmons, T. Kowalczyk,* H. Fliegl,* H. Shinokubo*
J. Am. Chem. Soc. 2021, 143, 10676−10685.
37. Non-Planar Perylene Bisimide Analogues with Inserted Carbonyl and Methylene Subunits
M. Odajima, K. Tajima, N. Fukui,* H. Shinokubo*
Angew. Chem. Int. Ed. 2021, 60, 15838−15843.

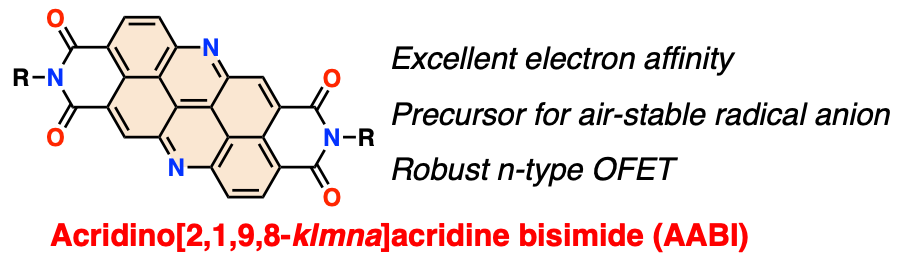
36. Acridino[2,1,9,8‐klmna]acridine Bisimides: An Electron‐Deficient π‐System for Robust Radical Anions and n‐Type Organic Semiconductors
K. Tajima, K. Matsuo, H. Yamada,* S. Seki,* N. Fukui,* H. Shinokubo*
Angew. Chem. Int. Ed. 2021, 60, 14060−14067.
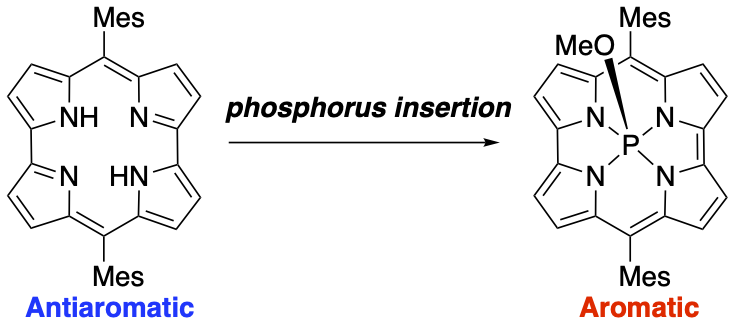
35. Synthesis and Properties of an 18π Aromatic Norcorrole P(V) Complex
T. Yoshida, S. A. Shafie, H. Kawashima, N. Fukui, H. Shinokubo*
Org. Lett. 2021, 23, 2826–2830.
34. Redox-induced reversible [2+2] cycloaddition of an etheno-fused diporphyrin
K. Miyagawa, I. Hisaki, N. Fukui, H. Shinokubo*
Chem. Sci. 2021, 12, 5224–5229.
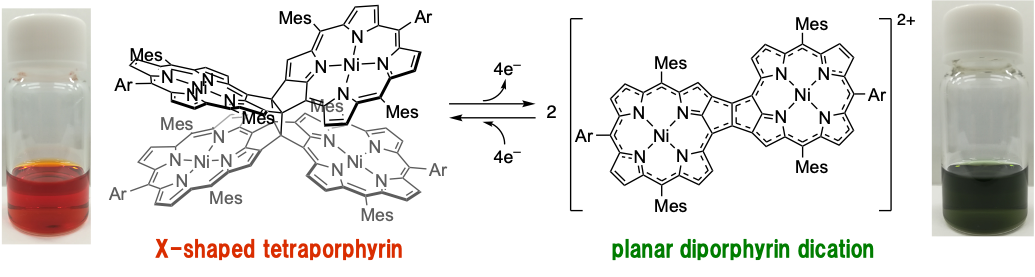
33. Dinaphtho[1,8-bc:1',8'-fg][1,5]dithiocine Bisimide
Y. Tanaka, K. Tajima, N. Fukui,* H. Shinokubo*
Asian J. Org. Chem. 2021, 10, 541–544.
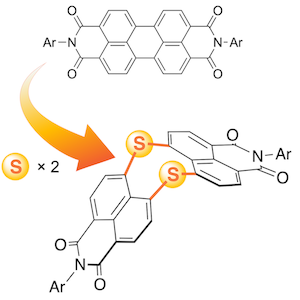
32. Dual Emission of a Free‐Base 5‐Oxaporphyrinium Cation from its cis‐ and trans‐NH Tautomers
A. Takiguchi, S. Kang, N. Fukui,* D. Kim,* H. Shinokubo*
Angew. Chem. Int. Ed. 2021, 64, 2915–2919.
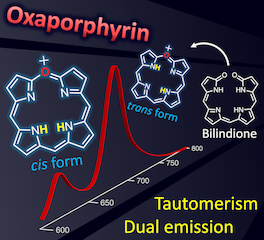
31. Synthesis and electron-transport property of stable antiaromatic NiII norcorrole with the smallest meso-substituent
S. Ukai, Y. Hee Koo, N. Fukui, S. Seki,* H. Shinokubo*
Dalton Trans. 2020, 49, 14383–14387.
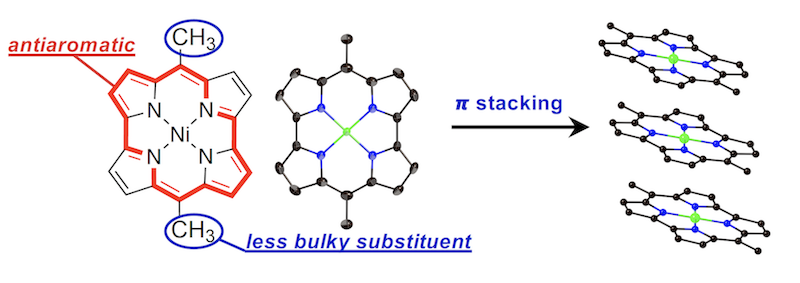
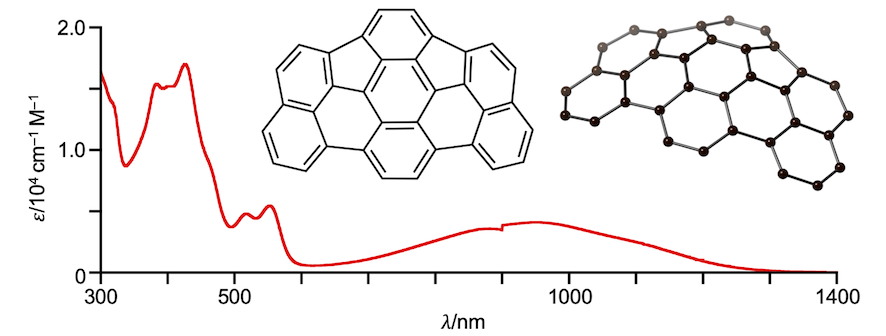
30. as-Indaceno[3,2,1,8,7,6-ghijklm]terrylene as a near-infrared absorbing C70-fragment
Y. Tanaka, N. Fukui,* H. Shinokubo*
Nat. Commun. 2020, 11, 3873.
Press Release (English, EurekAlert!)
Press Release (English)
Highlighted in Synfacts
Editors’ Highlights
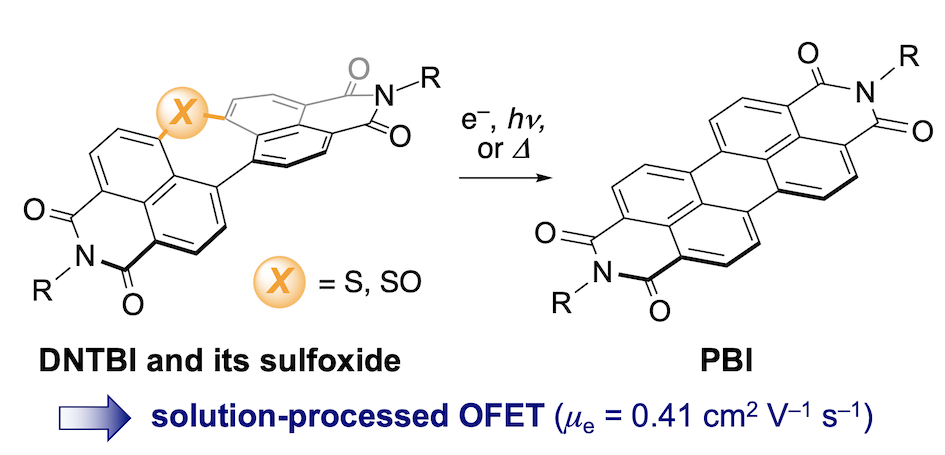
29. Dinaphthothiepine Bisimide and Its Sulfoxide: Soluble Precursors for Perylene Bisimide
S. Hayakawa, K. Matsuo, H. Yamada,* N. Fukui,* H. Shinokubo*
J. Am. Chem. Soc. 2020, 142, 11663−11668.
Highlighted in a newspaper(日刊工業新聞)
Highlighted in Synfacts
explanatory video (Japanese)
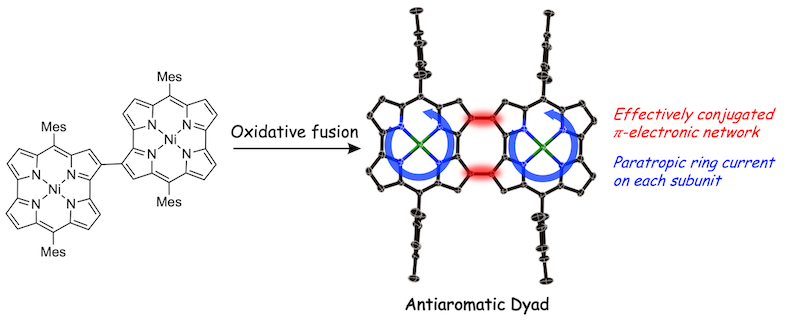
28. A 2-to-2′ 18-to-18′ doubly linked Ni(ii) norcorrole dimer: an effectively conjugated antiaromatic dyad
S.-Y. Liu, H. Kawashima, N. Fukui, H. Shinokubo*
Chem. Commun. 2020, 56, 6846−6849.
27. Reactions of Antiaromatic Norcorrole Ni(II) Complex with Carbenes
S.-Y. Liu, T. Fukuoka, N. Fukui, J.-Y. Shin, H. Shinokubo*
Org. Lett. 2020, 22, 4400–4403.

26. Synthesis and properties of 5-aza-15-thiaporphyrins
D. Yamashita, H. Omori, N. Fukui, H. Shinokubo*
J. Porphyr. Phthalocyanines. 2020, 24, 84−89.
25. Inserting Nitrogen: An Effective Concept to Create Nonplanar and Stimuli-Responsive Perylene Bisimide Analogues
S. Hayakawa, A. Kawasaki, Y. Hong, D. Uraguchi, T. Ooi, D. Kim,* T. Akutagawa,* N. Fukui,* H. Shinokubo*
J. Am. Chem. Soc. 2019, 141, 19807−19816.
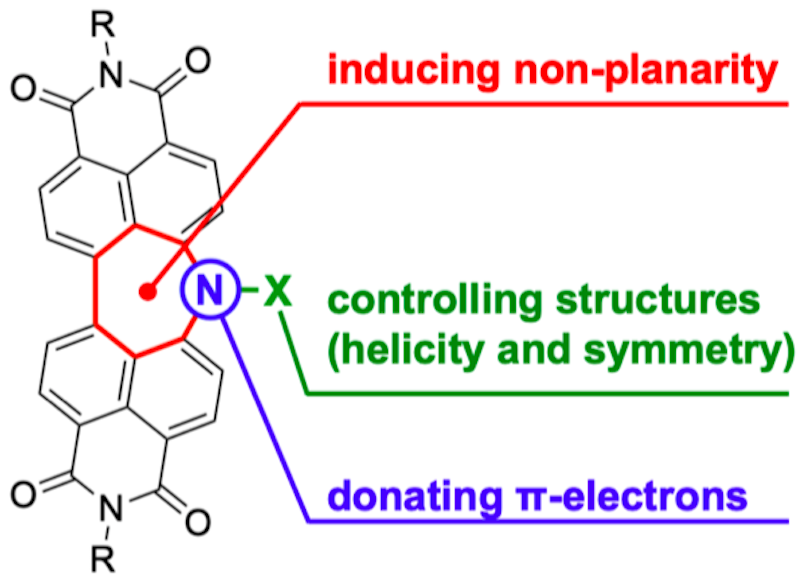
Press Release (Japanese)
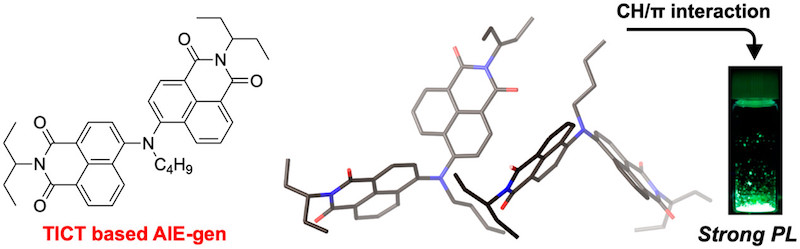
24. Aggregation-Induced Emission of Nitrogen-Bridged Naphthalene Monoimide Dimers
K. Tajima, N. Fukui,* H. Shinokubo*
Org. Lett. 2019, 21, 9516−9520.
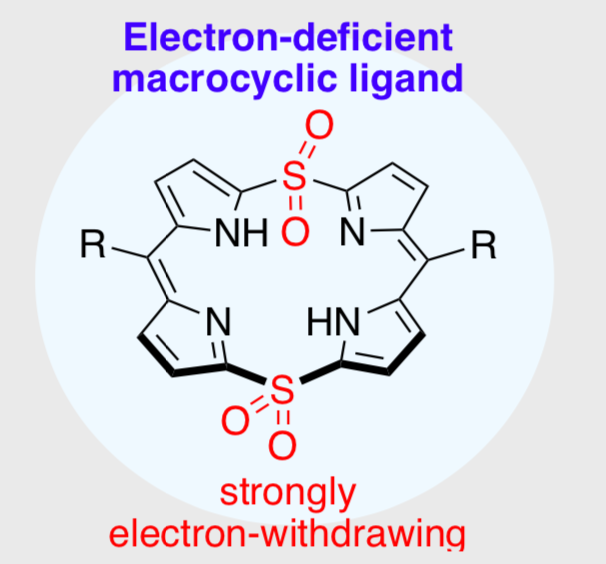
23. 5,5,15,15-Tetraoxo-5,15-Dithiaporphyrinas a Highly Electron-Deficient Porphyrinic Ligand
A. Yagi, T. Kondo, D. Yamashita, N. Wachi, H. Omori, N. Fukui, T. Ikeue, H. Shinokubo*
Chem. Eur. J. 2019, 25, 15580−15585.
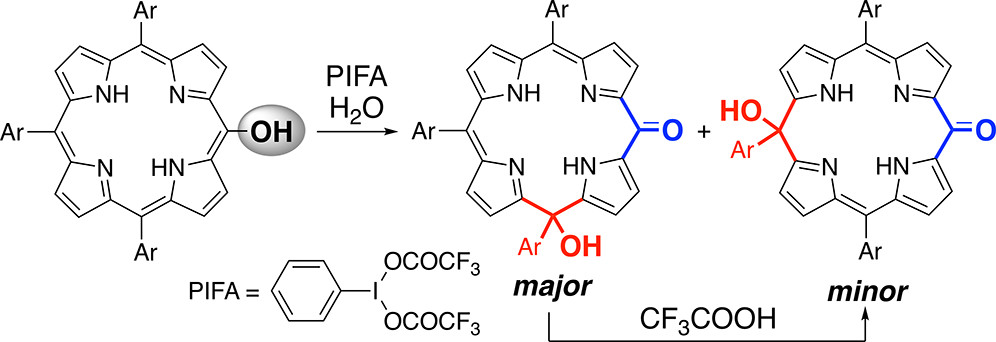
22. Synthesis of Hydroxyisooxophlorins by Oxidative Degradation of meso-Hydroxyporphyrins
A. Takiguchi, N. Fukui, H. Shinokubo,*
Org. Lett. 2019, 21, 3950-3953.
21. Synthesis of meso‐Alkyl‐Substituted Norcorrole Ni(II) Complexes and Conversion to 5‐Oxaporphyrins(2.0.1.0)
S. Liu, H. Tanaka, R. Nozawa, N. Fukui, H. Shinokubo*
Chem. Eur. J. 2019, 25, 7618-7622.
Selected as a Hot Paper
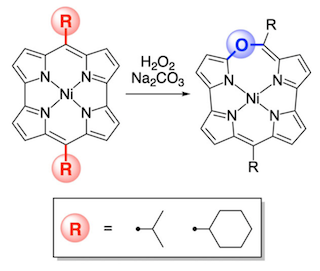
20. Macroscopically Anisotropic Structures Produced by Light-induced Solvothermal Assembly of Porphyrin Dimers)
Y. Yamamoto, Y. Nishimura, S. Tokonami, N. Fukui, T. Tanaka, A. Osuka, H. Yorimitsu, and T. Iida
Sci. Rep. 2018, 8, 11108.
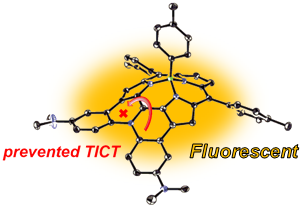
19. Diarylamine-Fused Subporphyrins: Proof of Twisted Intramolecular Charge Transfer (TICT) Mechanism
Koki Kise, Yongseok Hong, Norihito Fukui, Daiki Shimizu, Dongho Kim,* and Atsuhiro Osuka*
Chem. Eur. J. 2018, 24, 8306–8310.
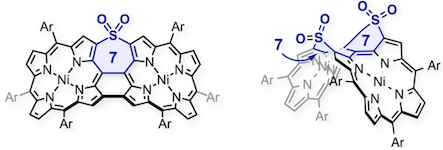
18. Singly and Doubly Sulfone-inserted Porphyrin Arch-Tape Dimers
Norihito Fukui and Atsuhiro Osuka*
Bull. Chem. Soc. Jpn. 2018, 91, 1131–1137.
Selected as a BCSJ Award Article
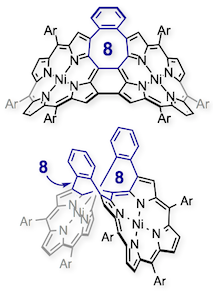
17. Singly and Doubly 1,2-Phenylene-Inserted Porphyrin Arch-Tape Dimers: Synthesis and Highly Contorted Structures
Norihito Fukui and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2018, 57, 6304–6308.
16. Metalation Control of Open-shell Character in meso-meso Linked Porphyrin meso-Oxy Radical Dimers
Yuta Jun-i, Norihito Fukui, Ko Furukawa,* and Atsuhiro Osuka*
Chem. Eur. J. 2018, 24, 1528–1532.

15. A meso-meso β-β β-β Triply Linked Subporphyrin Dimer
Yasuhiro Okuda, Norihito Fukui, Jinseok Kim, Hua-Wei Jiang, Graeme Copley, Masaaki Kitano, Dongho Kim,* and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2017, 56, 12317–12321.
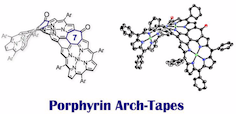
14. Porphyrin Arch-Tapes: Synthesis, Contorted Structures, and Full Conjugation
Norihito Fukui, Taeyon Kim, Dongho Kim,* and Atsuhiro Osuka*
J. Am. Chem. Soc. 2017, 139, 9075–9088.
Selected as a JACS Spotlight
Highlighted in Synfacts
13. Diphenylphosphine-Oxide-Fused and Diphenylphosphine-Fused Porphyrins: Synthesis, Tunable Electronic Properties, and Formation of Cofacial Dimers
Keisuke Fujimoto, Yuko Kasuga, Norihito Fukui, and Atsuhiro Osuka*
Chem. Eur. J. 2017, 23, 6741–6745.
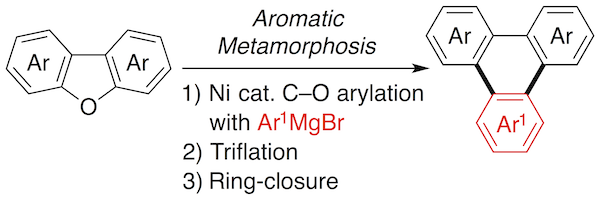
12. Aromatic Metamorphosis of Dibenzofurans into Triphenylenes Starting with Nickel-Catalyzed Ring-Opening C-O Arylation
Yuto Kurata, Sinya Otsuka, Norihito Fukui, Keisuke Nogi, Hideki Yorimitsu,* and Atsuhiro Osuka
Org. Lett. 2017, 19, 1274–1277.
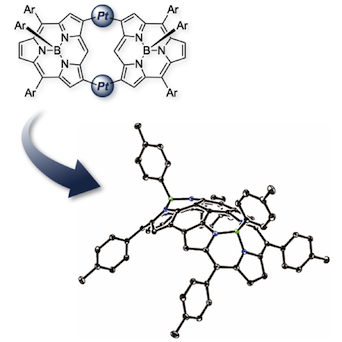
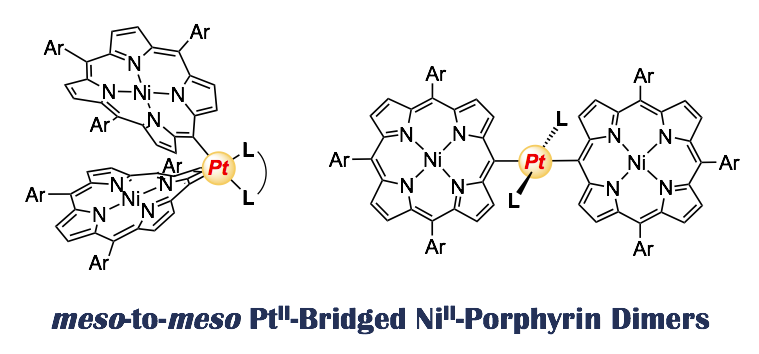
11. meso-to-meso PtII-bridged NiII-porphyrin dimers
Norihito Fukui, Hua-Wei Jiang, and Atsuhiro Osuka*
Org. Chem. Front. 2017, 4, 767–772.
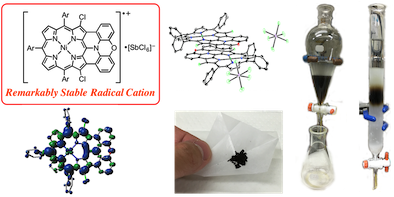
10. Highly planar diarylamine-fused porphyrins and their remarkably stable radical cations
Norihito Fukui, Wonhee Cha, Daiki Shimizu, Juwon Oh, Ko Furukawa,* Hideki Yorimitsu,* Dongho Kim,* and Atsuhiro Osuka*
Chem. Sci. 2017, 8, 189–199.
Highlighted in Synfacts
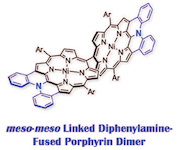
9. meso-meso Linked Diarylamine-Fused Porphyrin Dimers
Norihito Fukui, Hideki Yorimitsu,* and Atsuhiro Osuka*
Chem. Eur. J. 2016, 22, 18476–18483.
8. Pictet–Spengler Synthesis of Quinoline-Fused Porphyrins and Phenanthroline-Fused Diporphyrins
Ke Gao, Norihito Fukui, Seok Il Jung, Hideki Yorimitsu,* Dongho Kim,* and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2016, 55, 13038–13042.
Highlighted in Synfacts

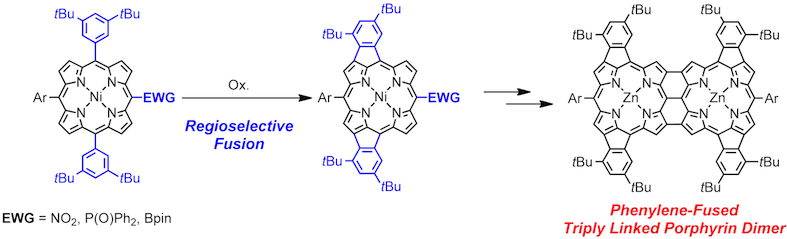
7. Regioselective phenylene-fusion reactions of Ni(II)-porphyrins controlled by an electron-withdrawing meso-substituent
Norihito Fukui, Seung-Kyu Lee, Kenichi Kato, Daiki Shimizu, Takayuki Tanaka, Sangsu Lee, Hideki Yorimitsu,* Dongho Kim,* and Atsuhiro Osuka*
Chem. Sci. 2016, 7, 4059–4066.
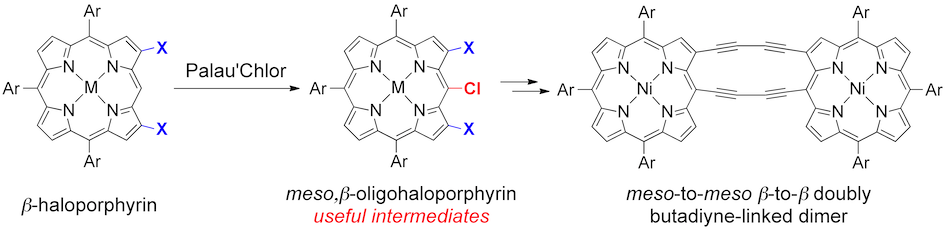
6. meso,β-Oligohaloporphyrins as Useful Synthetic Intermediates of Diphenylamine-Fused Porphyrin and meso-to-meso β-to-β Doubly Butadiyne-Bridged Diporphyrin
Norihito Fukui, Hideki Yorimitsu,* and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2015, 54, 6311–6314.
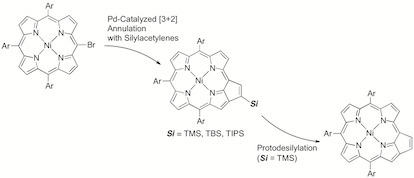
5. Palladium-Catalyzed [3+2] Annulation of meso-Bromoporphyrin with Silylacetylenes and Desilylation of 8a-Silyl-7,8-dehydropurpurin
Norihito Fukui, Seiji Arai, Hiroshi Shinokubo, Hideki Yorimitsu,* and Atsuhiro Osuka*
Heterocycles 2015, 90, 252–260.
4. Control of the conformational dynamics of meso-meso vinylene-bridged Zn(II) porphyrin dimers through diamine coordination
Minjung Son, Young Mo Sung, Sumito Tokuji, Norihito Fukui, Hideki Yorimitsu, Atsuhiro Osuka,* and Dongho Kim*
Chem. Commun. 2014, 50, 3078–3080.
3. Synthesis of 7,8-Dehydropurpurin Dimers and Their Conversion into Conformationally Constrained β-to-β Vinylene-Bridged Porphyrin Dimers
Norihito Fukui, Hideki Yorimitsu,* Jong Min Lim, Dongho Kim,* and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2014, 53, 4395–4398.
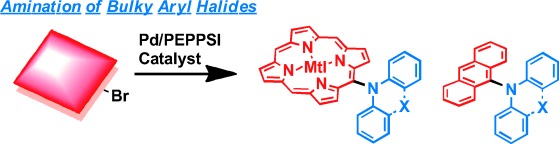
2. Amination of meso-Bromoporphyrins and 9-Haloanthracenes with Diarylamines Catalyzed by a Palladium–PEPPSI Complex
Yuko Suzuki, Norihito Fukui, Kei Murakami, Hideki Yorimitsu,* and Atsuhiro Osuka*
Asian J. Org. Chem. 2013, 2, 1066–1071.
Selected as the Front Cover
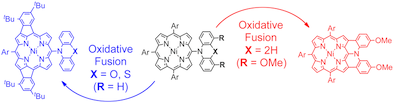
1. Oxidative Fusion Reactions of meso-(Diarylamino)porphyrins
Norihito Fukui, Won-Young Cha, Sangsu Lee, Sumito Tokuji, Dongho Kim,* Hideki Yorimitsu,* and Atsuhiro Osuka*
Angew. Chem. Int. Ed. 2013, 52, 9728–9732.
著書・総説
3. ポルフィリン類の直接官能基化
福井 識人、忍久保 洋
直接的芳香族カップリング反応の設計と応用(監修:三浦雅博、平野康次)、14章 2019, シーエムシー出版
2. Organic Transformations by the Hydrosilane-Alkoxide System
Norihito Fukui
J. Synth. Org. Chem. Jpn. 2019, 77, 512–513.
1. Embedding heteroatoms: an effective approach to create porphyrin-based functional materials
Norihito Fukui, Keisuke Fujimoto, Hideki Yorimitsu, and Atsuhiro Osuka*
Dalton Trans. 2017, 46, 13322–13341.