日本語(in Japanese)
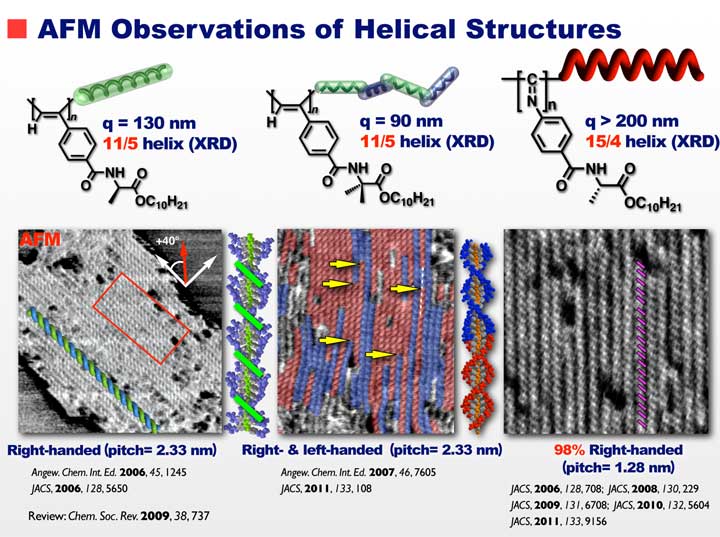
In the field of chiral polymers, in particular, helical polymers, there are lots of papers dealing with their synthesis and applications. However, evidence for a preferred-handed helix formation of synthetic helical polymers is usually obtained by CD or optical rotation. Based on X-ray diffraction (XRD) studies of oriented films or fibers derived from a few helical polymers, their helical structures have been proposed. However, these methods are not straightforward, and may not provide unambiguous helical structural information, and in particular, the helical sense. The determination of helical structures of helical polymers including the helical pitch and handedness by microscopy, a long-standing problem in polymer chemistry, has been for the first time achieved by us in 2006 for certain helical polymers using atomic force microscopy (AFM) coupled with solvent vapor exposures. We found rod-like helical polymers hierarchically self-assembled on graphite to form chiral two-dimensional (2D) helix-bundles with a controlled helicity, which enabled to directly observe the helical structures including helical pitch, handedness (right or left), helical-sense excess, and helical reversal as well. This method also made it possible to observe inversion of the macromolecular helices and merits further progress in helical polymers with specific structures and functions.